Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain
- PMID: 24086725
- PMCID: PMC3785459
- DOI: 10.1371/journal.pone.0076310
Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain
Abstract
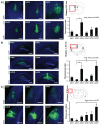
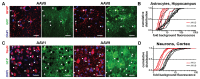
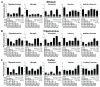
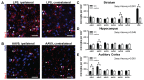
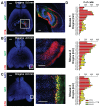
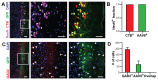
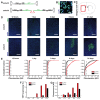
Recombinant Adeno-associated virus vectors (rAAV) are widely used for gene delivery and multiple naturally occurring serotypes have been harnessed to target cells in different tissues and organs including the brain. Here, we provide a detailed and quantitative analysis of the transduction profiles of rAAV vectors based on six of the most commonly used serotypes (AAV1, AAV2, AAV5, AAV6, AAV8, AAV9) that allows systematic comparison and selection of the optimal vector for a specific application. In our studies we observed marked differences among serotypes in the efficiency to transduce three different brain regions namely the striatum, hippocampus and neocortex of the mouse. Despite the fact that the analyzed serotypes have the general ability to transduce all major cell types in the brain (neurons, microglia, astrocytes and oligodendrocytes), the expression level of a reporter gene driven from a ubiquitous promoter varies significantly for specific cell type / serotype combinations. For example, rAAV8 is particularly efficient to drive transgene expression in astrocytes while rAAV9 appears well suited for the transduction of cortical neurons. Interestingly, we demonstrate selective retrograde transport of rAAV5 along axons projecting from the ventral part of the entorhinal cortex to the dentate gyrus. Furthermore, we show that self-complementing rAAV can be used to significantly decrease the time required for the onset of transgene expression in the mouse brain.
Conflict of interest statement
Figures
References
-
- Luo L, Callaway EM, Svoboda K (2008) Genetic dissection of neural circuits. Neuron 57: 634-660. doi:10.1016/j.neuron.2008.01.002. PubMed: 18341986. - DOI - PMC - PubMed
-
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L et al. (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358-362. doi:10.1038/nature09820. PubMed: 21389985. - DOI - PMC - PubMed
-
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS et al. (2012) Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484: 473-478. doi:10.1038/nature11039. PubMed: 22538608. - DOI - PMC - PubMed
-
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR et al. (2010) Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270-276. doi:10.1038/nature09553. PubMed: 21068836. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

