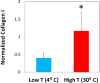
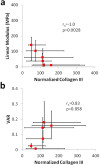
Distributions of types I, II and III collagen by region in the human supraspinatus tendon
- PMID: 24088220
- PMCID: PMC6056177
- DOI: 10.3109/03008207.2013.847096
Distributions of types I, II and III collagen by region in the human supraspinatus tendon
Abstract
The mechanical properties of the human supraspinatus tendon (SST) are highly heterogeneous and may reflect an important adaptive response to its complex, multiaxial loading environment. However, these functional properties are associated with a location-dependent structure and composition that have not been fully elucidated. Therefore, the objective of this study was to determine the concentrations of types I, II and III collagen in six distinct regions of the SST and compare changes in collagen concentration across regions with local changes in mechanical properties. We hypothesized that type I collagen content would be high throughout the tendon, type II collagen would be restricted to regions of compressive loading and type III collagen content would be high in regions associated with damage. We further hypothesized that regions of high type III collagen content would correspond to regions with low tensile modulus and a low degree of collagen alignment. Although type III collagen content was not significantly higher in regions that are frequently damaged, all other hypotheses were supported by our results. In particular, type II collagen content was highest near the insertion while type III collagen was inversely correlated with tendon modulus and collagen alignment. The measured increase in type II collagen under the coracoacromial arch provides evidence of adaptation to compressive loading in the SST. Moreover, the structure-function relationship between type III collagen content and tendon mechanics established in this study demonstrates a mechanism for altered mechanical properties in pathological tendons and provides a guideline for identifying therapeutic targets and pathology-specific biomarkers.
Conflict of interest statement
The authors report no conflict of interest and are alone responsible for the content and writing of this paper.
Figures
References
-
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. - PubMed
-
- Hunt SA, Kwon YW, Zuckerman JD. The rotator interval: anatomy, pathology, and strategies for treatment. J Am Acad Orthop Surg. 2007;15(4):218–27. - PubMed
-
- Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578–84. - PubMed
-
- Nakajima T, Rokuuma N, Hamada K, Tomatsu T, Fukuda H. Histologic and biomechanical characteristics of the supraspinatus tendon: Reference to rotator cuff tearing. J Shoulder Elbow Surg. 1994;3(2):79–87. - PubMed
-
- Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28(1):1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources