H+-type and OH- -type biological protonic semiconductors and complementary devices
- PMID: 24089083
- PMCID: PMC3789148
- DOI: 10.1038/srep02481
H+-type and OH- -type biological protonic semiconductors and complementary devices
Erratum in
-
Corrigendum: H(+)-type and OH(-)-type biological protonic semiconductors and complementary devices.Sci Rep. 2015 Jul 17;5:12118. doi: 10.1038/srep12118. Sci Rep. 2015. PMID: 26185057 Free PMC article. No abstract available.
Abstract
Proton conduction is essential in biological systems. Oxidative phosphorylation in mitochondria, proton pumping in bacteriorhodopsin, and uncoupling membrane potentials by the antibiotic Gramicidin are examples. In these systems, H(+) hop along chains of hydrogen bonds between water molecules and hydrophilic residues - proton wires. These wires also support the transport of OH(-) as proton holes. Discriminating between H(+) and OH(-) transport has been elusive. Here, H(+) and OH(-) transport is achieved in polysaccharide- based proton wires and devices. A H(+)- OH(-) junction with rectifying behaviour and H(+)-type and OH(-)-type complementary field effect transistors are demonstrated. We describe these devices with a model that relates H(+) and OH(-) to electron and hole transport in semiconductors. In turn, the model developed for these devices may provide additional insights into proton conduction in biological systems.
Figures
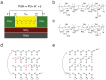
 going from left to right at the source and from right to left at the drain. The PdHx source and drain are connected to outside measurement electronics that measure the electronic current and complete the circuit. (b) Molecular structure of the H+-type proton conductor maleic chitosan, (c) Molecular structure of the OH− -type proton conductor proline chitosan. Degree of substitution defined as q/n + m determines the doping level. (d) Hop and turn Grotthuss mechanism for conductivity of H+ as hydronium ion along a proton wire. (e) Equivalent mechanism for OH− conductivity as proton hole along proton wire.
going from left to right at the source and from right to left at the drain. The PdHx source and drain are connected to outside measurement electronics that measure the electronic current and complete the circuit. (b) Molecular structure of the H+-type proton conductor maleic chitosan, (c) Molecular structure of the OH− -type proton conductor proline chitosan. Degree of substitution defined as q/n + m determines the doping level. (d) Hop and turn Grotthuss mechanism for conductivity of H+ as hydronium ion along a proton wire. (e) Equivalent mechanism for OH− conductivity as proton hole along proton wire.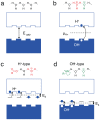
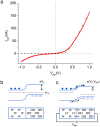
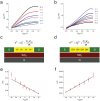
 (CG = gate capacitance per unit area, t = device thickness) (d) and
(CG = gate capacitance per unit area, t = device thickness) (d) and  . From simulations of dQ/dVgs, Cg = 3.85 × 10−4 F m−2. (e) (f) Plots of
. From simulations of dQ/dVgs, Cg = 3.85 × 10−4 F m−2. (e) (f) Plots of  as function of VGS and linear fit for the device in (a) and (b) respectively. For cross σ and charge density calculations the cross sectional area of the devices was derived from AFM and the cross sections were approximated to a rectangle with t = 66 nm for (a) and t = 160 nm (b) with the same widths as the actual devices. From the fit,
as function of VGS and linear fit for the device in (a) and (b) respectively. For cross σ and charge density calculations the cross sectional area of the devices was derived from AFM and the cross sections were approximated to a rectangle with t = 66 nm for (a) and t = 160 nm (b) with the same widths as the actual devices. From the fit,  and
and  .
.References
-
- DeCoursey T. E. Voltage-gated proton channels and other proton transfer pathways (vol 83, pg 475, 2003). Physiol. Rev. 83, 1067–1067 (2003). - PubMed
-
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 41, 445–502 (1966). - PubMed
-
- Morowitz H. J. Proton Semiconductors and Energy Transduction in Biological-Systems. Am. J. Physiol. 235, R99–R114 (1978). - PubMed
-
- Lanyi J. K. Bacteriorhodopsin. Annu. Rev. Physiol. 66, 665–688 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

