Knockdown of β-catenin with dicer-substrate siRNAs reduces liver tumor burden in vivo
- PMID: 24089139
- PMCID: PMC3978813
- DOI: 10.1038/mt.2013.233
Knockdown of β-catenin with dicer-substrate siRNAs reduces liver tumor burden in vivo
Abstract
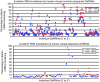
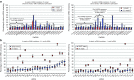
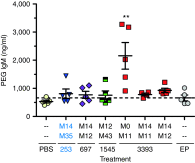
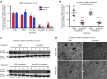
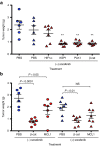
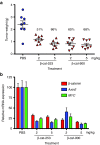
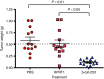
Despite progress in identifying molecular drivers of cancer, it has been difficult to translate this knowledge into new therapies, because many of the causal proteins cannot be inhibited by conventional small molecule therapeutics. RNA interference (RNAi), which uses small RNAs to inhibit gene expression, provides a promising alternative to reach traditionally undruggable protein targets by shutting off their expression at the messenger RNA (mRNA) level. Challenges for realizing the potential of RNAi have included identifying the appropriate genes to target and achieving sufficient knockdown in tumors. We have developed high-potency Dicer-substrate short-interfering RNAs (DsiRNAs) targeting β-catenin and delivered these in vivo using lipid nanoparticles, resulting in significant reduction of β-catenin expression in liver cancer models. Reduction of β-catenin strongly reduced tumor burden, alone or in combination with sorafenib and as effectively as DsiRNAs that target mitotic genes such as PLK1 and KIF11. β-catenin knockdown also strongly reduced the expression of β-catenin-regulated genes, including MYC, providing a potential mechanism for tumor inhibition. These results validate β-catenin as a target for liver cancer therapy and demonstrate the promise of RNAi in general and DsiRNAs in particular for reaching traditionally undruggable cancer targets.
Figures
Similar articles
-
Direct Pharmacological Inhibition of β-Catenin by RNA Interference in Tumors of Diverse Origin.Mol Cancer Ther. 2016 Sep;15(9):2143-54. doi: 10.1158/1535-7163.MCT-16-0309. Epub 2016 Jul 7. Mol Cancer Ther. 2016. PMID: 27390343 Free PMC article.
-
Inhibition of the Wnt/β-catenin signaling pathway improves the anti-tumor effects of sorafenib against hepatocellular carcinoma.Cancer Lett. 2016 Oct 10;381(1):58-66. doi: 10.1016/j.canlet.2016.07.013. Epub 2016 Jul 16. Cancer Lett. 2016. PMID: 27431312
-
The Effect of Dicer Knockout on RNA Interference Using Various Dicer Substrate Small Interfering RNA (DsiRNA) Structures.Genes (Basel). 2022 Feb 27;13(3):436. doi: 10.3390/genes13030436. Genes (Basel). 2022. PMID: 35327991 Free PMC article.
-
RNA interference: mechanisms, technical challenges, and therapeutic opportunities.Methods Mol Biol. 2015;1218:1-15. doi: 10.1007/978-1-4939-1538-5_1. Methods Mol Biol. 2015. PMID: 25319642 Review.
-
RNA-based therapeutics: an overview and prospectus.Cell Death Dis. 2022 Jul 23;13(7):644. doi: 10.1038/s41419-022-05075-2. Cell Death Dis. 2022. PMID: 35871216 Free PMC article. Review.
Cited by
-
Loss of β-catenin in adrenocortical cancer cells causes growth inhibition and reversal of epithelial-to-mesenchymal transition.Oncotarget. 2015 May 10;6(13):11421-33. doi: 10.18632/oncotarget.3222. Oncotarget. 2015. PMID: 25823656 Free PMC article.
-
Frontal Bone Healing Is Sensitive to Wnt Signaling Inhibition via Lentiviral-Encoded Beta-Catenin Short Hairpin RNA.Tissue Eng Part A. 2018 Aug 20;24(23-24):1742-52. doi: 10.1089/ten.TEA.2017.0465. Online ahead of print. Tissue Eng Part A. 2018. PMID: 29929440 Free PMC article.
-
Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides.Nucleic Acids Res. 2016 Jan 29;44(2):863-77. doi: 10.1093/nar/gkv1206. Epub 2015 Nov 17. Nucleic Acids Res. 2016. PMID: 26578588 Free PMC article.
-
Current development of targeted oligonucleotide-based cancer therapies: Perspective on HER2-positive breast cancer treatment.Cancer Treat Rev. 2016 Apr;45:19-29. doi: 10.1016/j.ctrv.2016.02.005. Epub 2016 Feb 22. Cancer Treat Rev. 2016. PMID: 26930249 Free PMC article. Review.
-
Multifunctional Role of Astrocyte Elevated Gene-1 (AEG-1) in Cancer: Focus on Drug Resistance.Cancers (Basel). 2021 Apr 9;13(8):1792. doi: 10.3390/cancers13081792. Cancers (Basel). 2021. PMID: 33918653 Free PMC article. Review.
References
-
- Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, et al. Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev. 2001;21:499–512. - PubMed
-
- Minna JD, Dowell J. Erlotinib hydrochloride. Nat Rev Drug Discov. 2005. pp. S14–S15. - PubMed
-
- Bollag G, Freeman S, Lyons JF, Post LE. Raf pathway inhibitors in oncology. Curr Opin Investig Drugs. 2003;4:1436–1441. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

