Retinoblastoma protein (RB1) controls fate determination in stem cells and progenitors of the mouse male germline
- PMID: 24089198
- PMCID: PMC4434987
- DOI: 10.1095/biolreprod.113.113159
Retinoblastoma protein (RB1) controls fate determination in stem cells and progenitors of the mouse male germline
Abstract
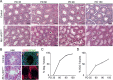
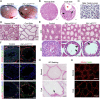
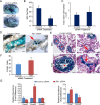
Continual spermatogenesis is the cornerstone of male fertility and relies on the actions of an undifferentiated spermatogonial population comprised of stem cells and progenitors. A foundational spermatogonial stem cell (SSC) pool is established during postnatal development that serves as a self-renewing reservoir from which progenitor spermatogonia arise that transiently amplify in number before committing to terminal differentiation. At present, the underlying molecular mechanisms governing these actions are undefined. Using conditional mutant mouse models, we investigated whether function of the undifferentiated spermatogonial population during postnatal life is influenced by the tumor suppressor protein RB1. Spermatogenesis initiates in mice with conditional inactivation of Rb1 in prospermatogonial precursors, but the germline is progressively lost upon aging due to impaired renewal of the undifferentiated spermatogonial population. In contrast, continual spermatogenesis is sustained following Rb1 inactivation in progenitor spermatogonia, but some cells transform into a carcinoma in situ-like state. Furthermore, knockdown of Rb1 abundance within primary cultures of wild-type undifferentiated spermatogonia impairs maintenance of the SSC pool, and some cells are invasive of the basement membrane after transplant into recipient testes, indicating acquisition of tumorigenic properties. Collectively, these findings indicate that RB1 plays an essential role in establishment of a self-renewing SSC pool and commitment to the spermatogenic lineage within progenitor spermatogonia.
Keywords: spermatogenesis; spermatogonia; spermatogonial stem cells; stem cells; testis.
Figures


Similar articles
-
Mechanisms regulating mammalian spermatogenesis and fertility recovery following germ cell depletion.Cell Mol Life Sci. 2019 Oct;76(20):4071-4102. doi: 10.1007/s00018-019-03201-6. Epub 2019 Jun 28. Cell Mol Life Sci. 2019. PMID: 31254043 Free PMC article. Review.
-
Stage-specific embryonic antigen 4 is a membrane marker for enrichment of porcine spermatogonial stem cells.Andrology. 2020 Nov;8(6):1923-1934. doi: 10.1111/andr.12870. Epub 2020 Aug 9. Andrology. 2020. PMID: 32691968
-
Single-cell transcriptome analyses reveal critical regulators of spermatogonial stem cell fate transitions.BMC Genomics. 2024 Feb 3;25(1):138. doi: 10.1186/s12864-024-10072-0. BMC Genomics. 2024. PMID: 38310206 Free PMC article.
-
Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation.Biol Reprod. 2009 Aug;81(2):293-301. doi: 10.1095/biolreprod.109.075960. Epub 2009 Apr 15. Biol Reprod. 2009. PMID: 19369648 Free PMC article.
-
Spermatogonial stem cell functions in physiological and pathological conditions.Curr Top Dev Biol. 2014;107:235-67. doi: 10.1016/B978-0-12-416022-4.00009-3. Curr Top Dev Biol. 2014. PMID: 24439809 Review.
Cited by
-
Developmental underpinnings of spermatogonial stem cell establishment.Andrology. 2020 Jul;8(4):852-861. doi: 10.1111/andr.12810. Epub 2020 May 24. Andrology. 2020. PMID: 32356598 Free PMC article. Review.
-
Paeoniflorin Promotes Ovarian Development in Mice by Activating Mitophagy and Preventing Oxidative Stress.Int J Mol Sci. 2024 Jul 30;25(15):8355. doi: 10.3390/ijms25158355. Int J Mol Sci. 2024. PMID: 39125927 Free PMC article.
-
Transcriptional control of stem cell fate by E2Fs and pocket proteins.Front Genet. 2015 Apr 28;6:161. doi: 10.3389/fgene.2015.00161. eCollection 2015. Front Genet. 2015. PMID: 25972892 Free PMC article. Review.
-
Diagnosing spermatogonial stemness.Biol Reprod. 2015 May;92(5):119. doi: 10.1095/biolreprod.115.129890. Epub 2015 Mar 25. Biol Reprod. 2015. PMID: 25810473 Free PMC article. No abstract available.
-
Cell-autonomous requirement for mammalian target of rapamycin (Mtor) in spermatogonial proliferation and differentiation in the mouse†.Biol Reprod. 2017 Apr 1;96(4):816-828. doi: 10.1093/biolre/iox022. Biol Reprod. 2017. PMID: 28379293 Free PMC article.
References
-
- De Rooij DG. Regulation of the proliferation of spermatogonial stem cells J Cell Sci Suppl 1988. 10 181– 194 - PubMed
-
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function Development 1991. 113 689– 699 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

