Paradoxical effects of heme arginate on survival of myocutaneous flaps
- PMID: 24089372
- PMCID: PMC3921308
- DOI: 10.1152/ajpregu.00240.2013
Paradoxical effects of heme arginate on survival of myocutaneous flaps
Abstract
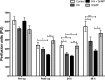
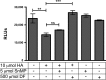
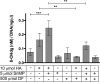
Ischemia reperfusion injury (IRI) contributes to partial flap and solid organ transplant failure. Heme-oxygenase 1 (HO-1) is an inducible, cytoprotective enzyme which protects against IRI in solid organ transplant models. Heme arginate (HA), a HO-1 inducer, is a promising, translatable, preconditioning agent. This study investigated the effects of preconditioning with HA on the clinical outcome of a myocutaneous IRI model. Forty male Lewis rats were randomized to intravenously receive 1) Control-NaCl, 2) HA, 3) HA and tin mesoporphyrin (SnMP), a HO-1 inhibitor; and 4) SnMP alone. Twenty-four hours later, an in situ transverse rectus abdominis myocutaneous flap was performed under isoflurane anesthesia. Viability of flaps was measured clinically and by laser-Doppler perfusion scanning. In vitro work on human epidermal keratinocytes (HEKa) assessed the effects of HA, SnMP, and the iron chelator desferrioxamine on 1) cytotoxicity, 2) intracellular reactive oxygen species (ROS) concentration, and 3) ROS-mediated DNA damage. In contrast to our hypothesis, HA preconditioning produced over 30% more flap necrosis at 48 h compared with controls (P = 0.02). HA-containing treatments produced significantly worse flap perfusion at all postoperative time points. In vitro work showed that HA is cytotoxic to keratinocytes. This cytotoxicity was independent of HO-1 and was mediated by the generation of ROS by free heme. In contrast to solid organ data, pharmacological preconditioning with HA significantly worsened clinical outcome, thus indicating that this is not a viable approach in free flap research.
Keywords: free tissue transfer; heme arginate; heme-oxygenase-1; ischemia reperfusion injury; myocutaneous flap.
Figures







Similar articles
-
Ischemic preconditioning improves the survival of skin and myocutaneous flaps in a rat model.Plast Reconstr Surg. 1998 Jul;102(1):140-50; discussion 151-2. doi: 10.1097/00006534-199807000-00022. Plast Reconstr Surg. 1998. PMID: 9655419
-
Heme Oxygenase Improves Renal Function by Potentiating Podocyte-Associated Proteins in Nω-Nitro-l-Arginine-Methyl Ester (l-NAME)-Induced Hypertension.Am J Hypertens. 2015 Jul;28(7):930-42. doi: 10.1093/ajh/hpu240. Epub 2014 Dec 12. Am J Hypertens. 2015. PMID: 25498996
-
Isoflurane preconditioning at clinically relevant doses induce protective effects of heme oxygenase-1 on hepatic ischemia reperfusion in rats.BMC Gastroenterol. 2011 Mar 31;11:31. doi: 10.1186/1471-230X-11-31. BMC Gastroenterol. 2011. PMID: 21453462 Free PMC article.
-
Heme oxygenase system in ischemia and reperfusion injury.Ann Transplant. 2004;9(1):84-7. Ann Transplant. 2004. PMID: 15478901 Review.
-
Cytoprotective effects of heme oxygenase in acute renal failure.Contrib Nephrol. 2005;148:70-85. doi: 10.1159/000086044. Contrib Nephrol. 2005. PMID: 15912028 Review.
Cited by
-
GRK2 deletion improves the function of skin flap following ischemia-reperfusion injury by regulating Drp1.Am J Transl Res. 2021 Jan 15;13(1):223-233. eCollection 2021. Am J Transl Res. 2021. PMID: 33527020 Free PMC article.
-
Heme oxygenase‑1 improves the survival of ischemic skin flaps (Review).Mol Med Rep. 2021 Apr;23(4):235. doi: 10.3892/mmr.2021.11874. Epub 2021 Feb 4. Mol Med Rep. 2021. PMID: 33537805 Free PMC article.
References
-
- Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 1790: 589–599, 2009 - PubMed
-
- Attuwaybi BO, Kozar RA, Moore-Olufemi SD, Sato N, Hassoun HT, Weisbrodt NW, Moore FA. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res 118: 53–57, 2004 - PubMed
-
- Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J 19: 1216–1219, 2005 - PubMed
-
- Balla J, Vercellotti GM, KN, Yachie A, Nagy E, Eaton JW, Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transpl 18: v8–v12, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

