Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism
- PMID: 24089484
- PMCID: PMC3787498
- DOI: 10.1523/JNEUROSCI.1175-13.2013
Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism
Abstract
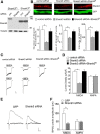
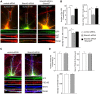
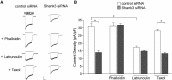
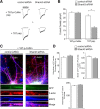
Shank3, which encodes a scaffolding protein at glutamatergic synapses, is a genetic risk factor for autism. In this study, we examined the impact of Shank3 deficiency on the NMDA-type glutamate receptor, a key player in cognition and mental illnesses. We found that knockdown of Shank3 with a small interfering RNA (siRNA) caused a significant reduction of NMDAR-mediated ionic or synaptic current, as well as the surface expression of NR1 subunits, in rat cortical cultures. The effect of Shank3 siRNA on NMDAR currents was blocked by an actin stabilizer, and was occluded by an actin destabilizer, suggesting the involvement of actin cytoskeleton. Since actin dynamics is regulated by the GTPase Rac1 and downstream effector p21-activated kinase (PAK), we further examined Shank3 regulation of NMDARs when Rac1 or PAK was manipulated. We found that the reducing effect of Shank3 siRNA on NMDAR currents was mimicked and occluded by specific inhibitors for Rac1 or PAK, and was blocked by constitutively active Rac1 or PAK. Immunocytochemical data showed a strong reduction of F-actin clusters after Shank3 knockdown, which was occluded by a PAK inhibitor. Inhibiting cofilin, the primary downstream target of PAK and a major actin depolymerizing factor, prevented Shank3 siRNA from reducing NMDAR currents and F-actin clusters. Together, these results suggest that Shank3 deficiency induces NMDAR hypofunction by interfering with the Rac1/PAK/cofilin/actin signaling, leading to the loss of NMDAR membrane delivery or stability. It provides a potential mechanism for the role of Shank3 in cognitive deficit in autism.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
