Where pain meets action in the human brain
- PMID: 24089498
- PMCID: PMC6618478
- DOI: 10.1523/JNEUROSCI.3135-12.2013
Where pain meets action in the human brain
Abstract
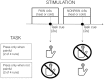
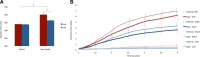
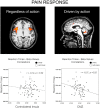
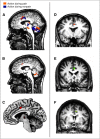
Pain's complex influence on behavior implies that it involves an action component, although little is known about how the human brain adaptively translates painful sensations into actions. The consistent activation of premotor and motor-related regions during pain, including the midcingulate cortex (MCC), raises the question of whether these areas contribute to an action component. In this fMRI experiment, we controlled for voluntary action-related processing during pain by introducing a motor task during painful or nonpainful stimulation. The MCC (particularly the caudal cingulate motor zone [CCZ]), motor cortex, thalamus, and cerebellum responded during action regardless of pain. Crucially, however, these regions did not respond to pain unless an action was performed. Reaction times were fastest during painful stimulation and correlated with CCZ activation. These findings are consistent with the results of an activation likelihood estimate meta-analysis in which activation across experiments involving pain, action execution, or action preparation (with a total of 4929 subjects) converged in a similar network. These findings suggest that specific motor-related areas, including the CCZ, play a vital role in the control and execution of context-sensitive behavioral responses to pain. In contrast, bilateral insular cortex responded to pain stimulation regardless of action.
Figures
Similar articles
-
The parietal operculum preferentially encodes heat pain and not salience.PLoS Biol. 2019 Aug 12;17(8):e3000205. doi: 10.1371/journal.pbio.3000205. eCollection 2019 Aug. PLoS Biol. 2019. PMID: 31404058 Free PMC article.
-
Functional brain imaging of trigeminal neuralgia.Eur J Pain. 2011 Feb;15(2):124-31. doi: 10.1016/j.ejpain.2010.06.006. Epub 2010 Jul 6. Eur J Pain. 2011. PMID: 20609605
-
Intracerebral ERD/ERS in voluntary movement and in cognitive visuomotor task.Prog Brain Res. 2006;159:311-30. doi: 10.1016/S0079-6123(06)59021-1. Prog Brain Res. 2006. PMID: 17071240
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
-
Activation likelihood estimation meta-analysis of motor-related neural activity after stroke.Neuroimage. 2012 Feb 1;59(3):2771-82. doi: 10.1016/j.neuroimage.2011.10.023. Epub 2011 Oct 17. Neuroimage. 2012. PMID: 22023742 Review.
Cited by
-
The endocannabinoid system in the brain undergoes long-lasting changes following neuropathic pain.iScience. 2024 Nov 22;27(12):111409. doi: 10.1016/j.isci.2024.111409. eCollection 2024 Dec 20. iScience. 2024. PMID: 39717086 Free PMC article. Review.
-
Top-Down Dysregulation-From ADHD to Emotional Instability.Front Behav Neurosci. 2016 May 23;10:70. doi: 10.3389/fnbeh.2016.00070. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27242456 Free PMC article.
-
Functional reorganisation in chronic pain and neural correlates of pain sensitisation: A coordinate based meta-analysis of 266 cutaneous pain fMRI studies.Neurosci Biobehav Rev. 2016 Sep;68:120-133. doi: 10.1016/j.neubiorev.2016.04.001. Epub 2016 May 7. Neurosci Biobehav Rev. 2016. PMID: 27168346 Free PMC article. Review.
-
Relation of gait measures with mild unilateral knee pain during walking using machine learning.Sci Rep. 2022 Dec 23;12(1):22200. doi: 10.1038/s41598-022-21142-2. Sci Rep. 2022. PMID: 36564397 Free PMC article.
-
Automated classification of pain perception using high-density electroencephalography data.J Neurophysiol. 2017 Feb 1;117(2):786-795. doi: 10.1152/jn.00650.2016. Epub 2016 Nov 30. J Neurophysiol. 2017. PMID: 27903639 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
