Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial
- PMID: 24089509
- PMCID: PMC3900540
- DOI: 10.2337/db13-0881
Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial
Abstract
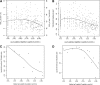
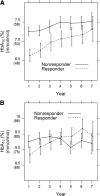
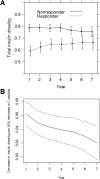
The Diabetes Control and Complications Trial established that a stimulated C-peptide concentration ≥0.2 nmol/L at study entry among subjects with up to a 5-year diabetes duration is associated with favorable metabolic and clinical outcomes over the subsequent 7 years of follow-up. Herein we further examine the association of both fasting and stimulated C-peptide numerical values with outcomes. In the intensive treatment group, for a 50% higher stimulated C-peptide on entry, such as from 0.10 to 0.15 nmol/L, HbA1c decreased by 0.07% (0.8 mmol/mol; P = 0.0003), insulin dose decreased by 0.0276 units/kg/day (P < 0.0001), hypoglycemia risk decreased by 8.2% (P < 0.0001), and the risk of sustained retinopathy was reduced by 25% (P = 0.0010), all in unadjusted analyses. Other than HbA1c, these effects remained significant after adjusting for the HbA1c on entry. While C-peptide was not significantly associated with the incidence of nephropathy, it was strongly associated with the albumin excretion rate. The fasting C-peptide had weaker associations with outcomes. As C-peptide decreased to nonmeasurable concentrations, the outcomes changed in a nearly linear manner, with no threshold or breakpoint. While preservation of stimulated C-peptide at ≥0.2 nmol/L has clinically beneficial outcomes, so also does an increase in the concentration of C-peptide across the range of values.
Figures
References
-
- Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001 [published correction appears in Diabetes 2004;53:1934]. Diabetes 2004;53:250–264 - PubMed
-
- The DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65:30–36 - PubMed
-
- The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 - PubMed
-
- Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

