Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation
- PMID: 24089632
- PMCID: PMC3786630
- DOI: 10.1136/ejhpharm-2012-000258
Equivalence and interchangeability of narrow therapeutic index drugs in organ transplantation
Abstract
The calcineurin inhibitors (CNIs), ciclosporin and tacrolimus, are the mainstay of immunosuppression in solid organ transplantation. Generic formulations of these drugs are now available. With increasing pressure on healthcare budgets and the consequent need to match health expectations to available resources, substitution with a generic product appears an attractive option to reduce costs. Approval of generic products differs from innovator drugs, and narrow therapeutic index drugs (NTIs; including CNIs) bring their own particular considerations. With NTIs, small variations in drug exposure could result in reduced immunosuppression or drug toxicity with potentially adverse effects on patient outcomes. NTIs are subject to stricter regulatory approval versus many other generic drugs. However, different generic formulations may still not necessarily be therapeutically equivalent in individuals, raising the possibility of significant differences in exposure between products. Although regional recommendations vary, many guidelines emphasise the need for NTI drug substitution to be initiated by the transplant physician, thus ensuring careful therapeutic monitoring and reduced negative patient impact. The need for therapeutic monitoring during generic substitution has important implications for the overall costs of generic treatment as these costs have to be factored in to the potential savings made from using generic formulations. The reduced acquisition costs of generic products may not necessarily translate into lower overall healthcare costs. This article examines the issue of equivalence and interchangeability of NTI drugs used in organ transplantation, the implications of the approval process for generic drugs on treatment efficacy and safety, and the effective management of substitutions between products.
Keywords: Transplant Medicine.
Figures

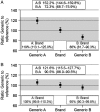
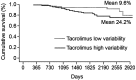

References
-
- Shapiro R, Young JB, Milford EL, et al. Immunosuppression: evolution in practice and trends, 1993–2003. Am J Transplant 2005;5:874–86 - PubMed
-
- Uber PA, Ross HJ, Zuckermann AO, et al. Generic drug immunosuppression in thoracic transplantation: an ISHLT educational advisory. J Heart Lung Transplant 2009;28:655–60 - PubMed
-
- European Medicines Agency Committee for Human Medicinal Products. Questions & Answers: positions on specific questions addressed to the Pharmacokinetics Working Party. EMA/618604/2008 Rev 5. September 2012
-
- Health Canada. 2012. Guidance document: comparative bioavailability standards: formulations used for systemic effects. Ottawa, Ontario: February.
-
- Kahan BD, Keown P, Levy GA, et al. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther 2002;24:330–50 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
