Soluble form of canine transferrin receptor inhibits canine parvovirus infection in vitro and in vivo
- PMID: 24089666
- PMCID: PMC3780538
- DOI: 10.1155/2013/172479
Soluble form of canine transferrin receptor inhibits canine parvovirus infection in vitro and in vivo
Abstract
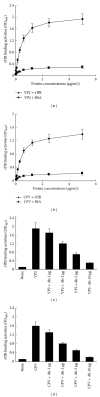
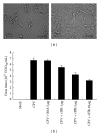
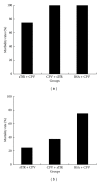
Canine parvovirus (CPV) disease is an acute, highly infectious disease threatening the dog-raising industry. So far there are no effective therapeutic strategies to control this disease. Although the canine transferrin receptor (TfR) was identified as a receptor for CPV infection, whether extracellular domain of TfR (called soluble TfR (sTfR)) possesses anti-CPV activities remains elusive. Here, we used the recombinant sTfR prepared from HEK293T cells with codon-optimized gene structure to investigate its anti-CPV activity both in vitro and in vivo. Our results indicated that codon optimization could significantly improve sTfR expression in HEK293T cells. The prepared recombinant sTfR possessed a binding activity to both CPV and CPV VP2 capsid proteins and significantly inhibited CPV infection of cultured feline F81 cells and decreased the mortality of CPV-infected dogs, which indicates that the sTfR has the anti-CPV activity both in vitro and in vivo.
Figures


Similar articles
-
Purified feline and canine transferrin receptors reveal complex interactions with the capsids of canine and feline parvoviruses that correspond to their host ranges.J Virol. 2006 Sep;80(17):8482-92. doi: 10.1128/JVI.00683-06. J Virol. 2006. PMID: 16912298 Free PMC article.
-
Evolutionary reconstructions of the transferrin receptor of Caniforms supports canine parvovirus being a re-emerged and not a novel pathogen in dogs.PLoS Pathog. 2012;8(5):e1002666. doi: 10.1371/journal.ppat.1002666. Epub 2012 May 3. PLoS Pathog. 2012. PMID: 22570610 Free PMC article.
-
Global distribution, cross-species transmission, and receptor binding of canine parvovirus-2: Risks and implications for humans.Sci Total Environ. 2024 Jun 20;930:172307. doi: 10.1016/j.scitotenv.2024.172307. Epub 2024 Apr 9. Sci Total Environ. 2024. PMID: 38599392
-
[Evolution and host variation of the canine parvovirus: molecular basis for the development of a new virus].Berl Munch Tierarztl Wochenschr. 2004 Mar-Apr;117(3-4):130-5. Berl Munch Tierarztl Wochenschr. 2004. PMID: 15046459 Review. German.
-
The Structures and Functions of Parvovirus Capsids and Missing Pieces: the Viral DNA and Its Packaging, Asymmetrical Features, Nonprotein Components, and Receptor or Antibody Binding and Interactions.J Virol. 2023 Jul 27;97(7):e0016123. doi: 10.1128/jvi.00161-23. Epub 2023 Jun 27. J Virol. 2023. PMID: 37367301 Free PMC article. Review.
Cited by
-
Profiling of Host Cell Response to Successive Canine Parvovirus Infection Based on Kinetic Proteomic Change Identification.Sci Rep. 2016 Jul 13;6:29560. doi: 10.1038/srep29560. Sci Rep. 2016. PMID: 27406444 Free PMC article.
-
Molecular cloning of chicken IL-7 and characterization of its antiviral activity against IBDV in vivo.Poult Sci. 2016 Nov 1;95(11):2647-2654. doi: 10.3382/ps/pew251. Epub 2016 Jul 27. Poult Sci. 2016. PMID: 27466431 Free PMC article.
-
Increased survival in puppies affected by Canine Parvovirus type II using an immunomodulator as a therapeutic aid.Sci Rep. 2021 Oct 6;11(1):19864. doi: 10.1038/s41598-021-99357-y. Sci Rep. 2021. PMID: 34615970 Free PMC article.
-
Transcriptome profiling indicating canine parvovirus type 2a as a potential immune activator.Virus Genes. 2016 Dec;52(6):768-779. doi: 10.1007/s11262-016-1363-5. Epub 2016 Jun 23. Virus Genes. 2016. PMID: 27339228 Free PMC article.
-
Nickel induces interleukin-1β secretion via the NLRP3-ASC-caspase-1 pathway.Inflammation. 2014 Apr;37(2):457-66. doi: 10.1007/s10753-013-9759-z. Inflammation. 2014. PMID: 24158569
References
-
- Parrish CR, O’Connell PH, Evermann JF, Carmichael LE. Natural variation of canine parvovirus. Science. 1985;230(4729):1046–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

