Pharmacophore modeling and docking studies on some nonpeptide-based caspase-3 inhibitors
- PMID: 24089669
- PMCID: PMC3780516
- DOI: 10.1155/2013/306081
Pharmacophore modeling and docking studies on some nonpeptide-based caspase-3 inhibitors
Abstract
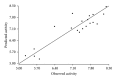
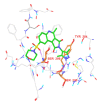
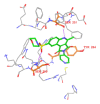
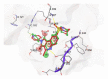
Neurodegenerative disorders are major consequences of excessive apoptosis caused by a proteolytic enzyme known as caspase-3. Therefore, caspase-3 inhibition has become a validated therapeutic approach for neurodegenerative disorders. We performed pharmacophore modeling on some synthetic derivatives of caspase-3 inhibitors (pyrrolo[3,4-c]quinoline-1,3-diones) using PHASE 3.0. This resulted in the common pharmacophore hypothesis AAHRR.6 which might be responsible for the biological activity: two aromatic rings (R) mainly in the quinoline nucleus, one hydrophobic (H) group (CH₃), and two acceptor (A) groups (-C=O). After identifying a valid hypothesis, we also developed an atom-based 3D-QSAR model applying the PLS algorithm. The developed model was statistically robust (q² = 0.53; pred_r² = 0.80). Additionally, we have performed molecular docking studies, cross-validated our results, and gained a deeper insight into its molecular recognition process. Our developed model may serve as a query tool for future virtual screening and drug designing for this particular target.
Figures




Similar articles
-
Identification of potent caspase-3 inhibitors for treatment of multi- neurodegenerative diseases using pharmacophore modeling and docking approaches.CNS Neurol Disord Drug Targets. 2014;13(8):1346-53. doi: 10.2174/1871527313666141023120843. CNS Neurol Disord Drug Targets. 2014. PMID: 25345515
-
A combination of pharmacophore modeling, atom-based 3D-QSAR, molecular docking and molecular dynamics simulation studies on PDE4 enzyme inhibitors.J Biomol Struct Dyn. 2016 Nov;34(11):2481-92. doi: 10.1080/07391102.2015.1119732. Epub 2016 Apr 12. J Biomol Struct Dyn. 2016. PMID: 26587754
-
Multi-Pharmacophore Modeling of Caspase-3 Inhibitors using Crystal, Dock and Flexible Conformation Schemes.Comb Chem High Throughput Screen. 2018;21(1):26-40. doi: 10.2174/1386207321666180102114917. Comb Chem High Throughput Screen. 2018. PMID: 29295689
-
Pharmacophore modeling, virtual screening and 3D-QSAR studies of 5-tetrahydroquinolinylidine aminoguanidine derivatives as sodium hydrogen exchanger inhibitors.Bioorg Med Chem Lett. 2012 Jun 1;22(11):3758-65. doi: 10.1016/j.bmcl.2012.04.012. Epub 2012 Apr 13. Bioorg Med Chem Lett. 2012. PMID: 22546667
-
Pharmacophore generation, atom-based 3D-QSAR, molecular docking and molecular dynamics simulation studies on benzamide analogues as FtsZ inhibitors.J Biomol Struct Dyn. 2018 Sep;36(12):3218-3230. doi: 10.1080/07391102.2017.1384401. Epub 2017 Oct 11. J Biomol Struct Dyn. 2018. PMID: 28938860
Cited by
-
Screening of caspase-3 inhibitors from natural molecule database using e-pharmacophore and docking studies.Bioinformation. 2019 Mar 31;15(4):240-245. doi: 10.6026/97320630015240. eCollection 2019. Bioinformation. 2019. PMID: 31285640 Free PMC article.
-
Implication of Caspase-3 as a Common Therapeutic Target for Multineurodegenerative Disorders and Its Inhibition Using Nonpeptidyl Natural Compounds.Biomed Res Int. 2015;2015:379817. doi: 10.1155/2015/379817. Epub 2015 May 4. Biomed Res Int. 2015. PMID: 26064904 Free PMC article.
-
Machine Learning From Molecular Dynamics Trajectories to Predict Caspase-8 Inhibitors Against Alzheimer's Disease.Front Pharmacol. 2019 Jul 12;10:780. doi: 10.3389/fphar.2019.00780. eCollection 2019. Front Pharmacol. 2019. PMID: 31354494 Free PMC article.
-
Current and emerging therapeutic targets of alzheimer's disease for the design of multi-target directed ligands.Medchemcomm. 2019 Oct 16;10(12):2052-2072. doi: 10.1039/c9md00337a. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32206241 Free PMC article. Review.
References
-
- Wellington CL, Hayden MR. Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clinical Genetics. 2000;57(1):1–10. - PubMed
-
- Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circulation Research. 1998;82(11):1111–1129. - PubMed
-
- Hoglen NC, Chen L, Fisher CD, Hirakawa BP, Groessl T, Contreras PC. Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]-4-oxo-5-(2,3,5, 6-tetrafluoro-phenoxy)-pentanoic acid): a liver-targeted caspase inhibitor. Journal of Pharmacology and Experimental Therapeutics. 2004;309(2):634–640. - PubMed
-
- Jacobson MD. Anti-apoptosis therapy: a way of treating neural degeneration? Current Biology. 1998;8(12):R418–R421. - PubMed
-
- Mohr A, Zwacka RM. In situ trapping of initiator caspases reveals intermediate surprises. Cell Biology International. 2007;31(5):526–530. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

