Lupus-like oral mucosal lesions in mercury-induced autoimmune response in Brown Norway rats
- PMID: 24089704
- PMCID: PMC3852543
- DOI: 10.1186/1471-2172-14-47
Lupus-like oral mucosal lesions in mercury-induced autoimmune response in Brown Norway rats
Abstract
Background: Administration of mercury at nontoxic doses induces systemic autoimmune disease in Brown Norway (BN) rats. The pathogenesis of lupus-like oral mucosal lesion by mercury-induced autoimmunity is still unclear, even though the oral mucosa is observed to be commonly affected in mercury-treated BN rats. In this study, we investigated the immunopathology of lupus-like oral mucosal lesions in a model of mercury-induced systemic autoimmunity.
Methods: Brown Norway male rats were injected subcutaneously with either phosphate-buffered saline (control) or mercury at a dose of 1.0 mg per kilogram of body weight on days 0, 3, 5, and 7. Blood, kidney, and tongue samples were taken at various timepoints for evaluation by immunohistochemistry, RT-PCR, and lupus band test (LBT).
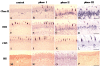
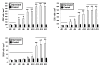
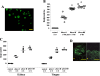
Results: Oral mucosal lesions were classified according to three consecutive temporal phases on the basis of infiltration of immunocompetent cells as follows: (phase I) infiltration of MHC class II+ dendritic cells (DC) and macrophages; (phase II) addition of ED1+ macrophage infiltrates; and (phase III) focal infiltration of pan T cells following increased infiltration of DC and macrophages. Dense infiltration of DC and macrophages was observed in the basement membrane (BM) zone of the oral epithelium. Tissue expression of IL-4 mRNA was detected in early lesions (phase I), suggesting that locally produced IL-4 may be responsible for Th2-mediated immune response. A linear and continuous smooth pattern of fluorescence was observed in the oral epithelial BM in addition to renal glomeruli, indicating immune complex deposits.
Conclusions: Local autoimmune responses are involved in the pathogenesis of mercury-induced lupus-like lesions of the oral mucosa.
Figures



Similar articles
-
Contact stomatitis to mercury associated with spontaneous mononuclear cell infiltrates in brown Norway (BN) rats with HgCl2-induced autoimmunity.J Oral Pathol Med. 1994 Nov;23(10):441-5. doi: 10.1111/j.1600-0714.1994.tb00441.x. J Oral Pathol Med. 1994. PMID: 7861329
-
Histochemical visualization of mercury in the oral mucosa, salivary and lacrimal glands of BN rats with HgCl2-induced autoimmunity.Exp Toxicol Pathol. 1994 Oct;46(4-5):329-34. doi: 10.1016/S0940-2993(11)80112-0. Exp Toxicol Pathol. 1994. PMID: 7894244
-
Induction of epithelial migration of lymphocytes by intercellular adhesion molecule-1 in a rat model of oral mucosal graft-versus-host disease.Histol Histopathol. 2011 Jun;26(6):725-33. doi: 10.14670/HH-26.725. Histol Histopathol. 2011. PMID: 21472687
-
Th2 and Th1 autoreactive anti-class II cell lines in the rat suppress or induce autoimmunity.J Autoimmun. 1996 Apr;9(2):221-6. doi: 10.1006/jaut.1996.0027. J Autoimmun. 1996. PMID: 8738966 Review.
-
[Immune glomerulopathies of toxic origin. Possible mechanisms of induction].Nephrologie. 1989;10(3):103-7. Nephrologie. 1989. PMID: 2691904 Review. French.
Cited by
-
Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius.Environ Sci Pollut Res Int. 2017 Mar;24(8):7555-7564. doi: 10.1007/s11356-016-8329-y. Epub 2017 Jan 23. Environ Sci Pollut Res Int. 2017. PMID: 28116627
-
Clinicopathological features and long-term prognosis of glomerular diseases associated with mercury-containing cosmetics.J Nephrol. 2023 Jun;36(5):1401-1407. doi: 10.1007/s40620-023-01606-y. Epub 2023 Apr 15. J Nephrol. 2023. PMID: 37060438
References
-
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich S. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

