Postmortem brain: an underutilized substrate for studying severe mental illness
- PMID: 24091486
- PMCID: PMC3857666
- DOI: 10.1038/npp.2013.239
Postmortem brain: an underutilized substrate for studying severe mental illness
Erratum in
-
Postmortem brain: an underutilized substrate for studying severe mental illness.Neuropsychopharmacology. 2015 Mar 13;40(5):1307. doi: 10.1038/npp.2014.337. Neuropsychopharmacology. 2015. PMID: 25767083 Free PMC article. No abstract available.
Abstract
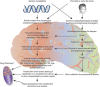
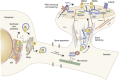
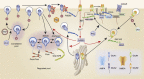
We propose that postmortem tissue is an underutilized substrate that may be used to translate genetic and/or preclinical studies, particularly for neuropsychiatric illnesses with complex etiologies. Postmortem brain tissues from subjects with schizophrenia have been extensively studied, and thus serve as a useful vehicle for illustrating the challenges associated with this biological substrate. Schizophrenia is likely caused by a combination of genetic risk and environmental factors that combine to create a disease phenotype that is typically not apparent until late adolescence. The complexity of this illness creates challenges for hypothesis testing aimed at understanding the pathophysiology of the illness, as postmortem brain tissues collected from individuals with schizophrenia reflect neuroplastic changes from a lifetime of severe mental illness, as well as treatment with antipsychotic medications. While there are significant challenges with studying postmortem brain, such as the postmortem interval, it confers a translational element that is difficult to recapitulate in animal models. On the other hand, data derived from animal models typically provide specific mechanistic and behavioral measures that cannot be generated using human subjects. Convergence of these two approaches has led to important insights for understanding molecular deficits and their causes in this illness. In this review, we discuss the problem of schizophrenia, review the common challenges related to postmortem studies, discuss the application of biochemical approaches to this substrate, and present examples of postmortem schizophrenia studies that illustrate the role of the postmortem approach for generating important new leads for understanding the pathophysiology of severe mental illness.
Figures

References
-
- Abekawa T, Ito K, Nakagawa S, Nakato Y, Koyama T. Olanzapine and risperidone block a high dose of methamphetamine-induced schizophrenia-like behavioral abnormalities and accompanied apoptosis in the medial prefrontal cortex. Schizophr Res. 2008;101:84–94. - PubMed
-
- Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry. 2000;47:361–370. - PubMed
-
- Alda M, Ahrens B, Lit W, Dvorakova M, Labelle A, Zvolsky P, et al. Age of onset in familial and sporadic schizophrenia. Acta Psychiatr Scand. 1996;93:447–450. - PubMed
-
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

