Lithium chloride attenuates cell death in oculopharyngeal muscular dystrophy by perturbing Wnt/β-catenin pathway
- PMID: 24091664
- PMCID: PMC3824652
- DOI: 10.1038/cddis.2013.342
Lithium chloride attenuates cell death in oculopharyngeal muscular dystrophy by perturbing Wnt/β-catenin pathway
Abstract
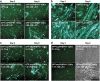
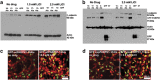
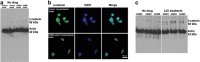
Expansion of polyalanine tracts causes at least nine inherited human diseases. Among these, a polyalanine tract expansion in the poly (A)-binding protein nuclear 1 (expPABPN1) causes oculopharyngeal muscular dystrophy (OPMD). So far, there is no treatment for OPMD patients. Developing drugs that efficiently sustain muscle protection by activating key cell survival mechanisms is a major challenge in OPMD research. Proteins that belong to the Wnt family are known for their role in both human development and adult tissue homeostasis. A hallmark of the Wnt signaling pathway is the increased expression of its central effector, beta-catenin (β-catenin) by inhibiting one of its upstream effector, glycogen synthase kinase (GSK)3β. Here, we explored a pharmacological manipulation of a Wnt signaling pathway using lithium chloride (LiCl), a GSK-3β inhibitor, and observed the enhanced expression of β-catenin protein as well as the decreased cell death normally observed in an OPMD cell model of murine myoblast (C2C12) expressing the expanded and pathogenic form of the expPABPN1. Furthermore, this effect was also observed in primary cultures of mouse myoblasts expressing expPABPN1. A similar effect on β-catenin was also observed when lymphoblastoid cells lines (LCLs) derived from OPMD patients were treated with LiCl. We believe manipulation of the Wnt/β-catenin signaling pathway may represent an effective route for the development of future therapy for patients with OPMD.
Figures




References
-
- Dion P, Shanmugam V, Gaspar C, Messaed C, Meijer I, Toulouse A, et al. Transgenic expression of an expanded (GCG)13 repeat PABPN1 leads to weakness and coordination defects in mice. Neurobiol Dis. 2005;18:528–536. - PubMed
-
- Boukriche Y, Maisonobe T, Masson C. Neurogenic involvement in a case of oculopharyngeal muscular dystrophy. Muscle Nerve. 2002;25:98–101. - PubMed
-
- Schober R, Kress W, Grahmann F, Kellermann S, Baum P, Gunzel S, et al. Unusual triplet expansion associated with neurogenic changes in a family with oculopharyngeal muscular dystrophy. Neuropathology. 2001;21:45–52. - PubMed
-
- Brais B, Rouleau GA, Bouchard JP, Fardeau M, Tome FM. Oculopharyngeal muscular dystrophy. Semin Neurol. 1999;19:59–66. - PubMed
-
- Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

