Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease
- PMID: 24091674
- PMCID: PMC3824657
- DOI: 10.1038/cddis.2013.347
Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease
Abstract
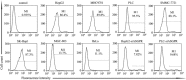
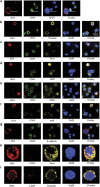
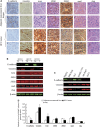
The presence of circulating tumor cells (CTCs) in peripheral blood is associated with metastasis and prognosis in hepatocellular carcinoma (HCC) patients. The epithelial-mesenchymal transition (EMT) has a pivotal role in tumor invasion and dissemination. To identify more sensitive biomarkers for evaluating metastasis and prognosis, we investigated the expression of EMT markers, including vimentin, twist, ZEB1, ZEB2, snail, slug and E-cadherin in CTCs, primary HCC tumors and adjacent non-tumoral liver tissues. After isolating viable CTCs from the peripheral blood of HCC patients using asialoglycoprotein receptors (ASGPRs), the CTCs were identified with immunofluorescence staining. CTCs were detected in the peripheral blood obtained from 46 of 60 (76.7%) HCC patients. Triple-immunofluorescence staining showed that twist and vimentin expression could be detected in CTCs obtained from 39 (84.8%) and 37 (80.4%) of the 46 patients, respectively. The expression of both twist and vimentin in CTCs was significantly correlated with portal vein tumor thrombus. Coexpression of twist and vimentin in CTCs could be detected in 32 (69.6%) of the 46 patients and was highly correlated with portal vein tumor thrombus, TNM classification and tumor size. Quantitative fluorescence western blot analysis revealed that the expression levels of E-cadherin, vimentin and twist in HCC tumors were significantly associated with the positivity of isolated CTCs (P=0.013, P=0.012, P=0.009, respectively). However, there was no significant difference in ZEB1, ZEB2, snail and slug expression levels in CTCs, primary HCC tumors and adjacent non-tumoral liver tissues across samples with regard to the clinicopathological parameters. Our results demonstrate that the EMT has a role in promoting the blood-borne dissemination of primary HCC cells, and the twist and vimentin expression levels in CTCs could serve as promising biomarkers for evaluating metastasis and prognosis in HCC patients.
Figures

References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. - PubMed
-
- Vogel I, Kalthoff H. Disseminated tumour cells. Their detection and significance for prognosis of gastrointestinal and pancreatic carcinomas. Virchows Arch. 2001;439:109–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

