Switch from protective to adverse inflammation during influenza: viral determinants and hemostasis are caught as culprits
- PMID: 24091817
- PMCID: PMC11114008
- DOI: 10.1007/s00018-013-1479-x
Switch from protective to adverse inflammation during influenza: viral determinants and hemostasis are caught as culprits
Abstract
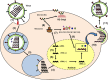
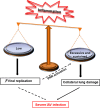
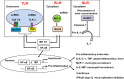
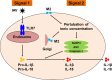
Influenza viruses cause acute respiratory infections, which are highly contagious and occur as seasonal epidemic and sporadic pandemic outbreaks. Innate immune response is activated shortly after infection with influenza A viruses (IAV), affording effective protection of the host. However, this response should be tightly regulated, as insufficient inflammation may result in virus escape from immunosurveillance. In contrast, excessive inflammation may result in bystander lung tissue damage, loss of respiratory capacity, and deterioration of the clinical outcome of IAV infections. In this review, we give a comprehensive overview of the innate immune response to IAV infection and summarize the most important findings on how the host can inappropriately respond to influenza.
Figures


References
-
- Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol. 2012;2(3):276–286. - PubMed
-
- Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3(8):591–600. - PubMed
-
- Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. 5. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1647–1689.
-
- Lamb RAKR. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, editors. Fields virology. Philadelphia: Lippincott Williams and Wilkins, Philadelphia; 2001. pp. 1487–1531.
-
- Moules V, Terrier O, Yver M, Riteau B, Moriscot C, Ferraris O, Julien T, Giudice E, Rolland JP, Erny A, Bouscambert-Duchamp M, Frobert E, Rosa-Calatrava M, Pu Lin Y, Hay A, Thomas D, Schoehn G, Lina B. Importance of viral genomic composition in modulating glycoprotein content on the surface of influenza virus particles. Virology. 2011;414(1):51–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

