Recent advances in understanding enteric pathogenic Escherichia coli
- PMID: 24092857
- PMCID: PMC3811233
- DOI: 10.1128/CMR.00022-13
Recent advances in understanding enteric pathogenic Escherichia coli
Abstract
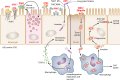
Although Escherichia coli can be an innocuous resident of the gastrointestinal tract, it also has the pathogenic capacity to cause significant diarrheal and extraintestinal diseases. Pathogenic variants of E. coli (pathovars or pathotypes) cause much morbidity and mortality worldwide. Consequently, pathogenic E. coli is widely studied in humans, animals, food, and the environment. While there are many common features that these pathotypes employ to colonize the intestinal mucosa and cause disease, the course, onset, and complications vary significantly. Outbreaks are common in developed and developing countries, and they sometimes have fatal consequences. Many of these pathotypes are a major public health concern as they have low infectious doses and are transmitted through ubiquitous mediums, including food and water. The seriousness of pathogenic E. coli is exemplified by dedicated national and international surveillance programs that monitor and track outbreaks; unfortunately, this surveillance is often lacking in developing countries. While not all pathotypes carry the same public health profile, they all carry an enormous potential to cause disease and continue to present challenges to human health. This comprehensive review highlights recent advances in our understanding of the intestinal pathotypes of E. coli.
Figures


References
-
- Escherich T, Bettelheim KS. 1988. The intestinal bacteria of the neonate and breast-fed infant. Rev. Infect. Dis. 10:1220–1225 - PubMed
-
- Cowan ST. 1954. A review of names for coliform organisms. Int. Bull. Bacteriol. Nomencl. Taxon. 4:119–124
-
- Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 - PubMed
-
- Croxen MA, Finlay BB. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8:26–38 - PubMed
-
- World Health Organization 2012. World health statistics 2012 WHO Press, Geneva, Switzerland
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

