TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats
- PMID: 24092879
- PMCID: PMC3882051
- DOI: 10.1242/dmm.013326
TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats
Abstract
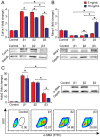
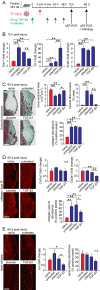
Transforming growth factor (TGF)-β1 and TGF-β3 have been reported to exert differential effects on wound healing, and possibly even account for tissue-specific differences in scar formation. Scarring is particularly detrimental in the vocal fold mucosa (VFM), where destruction of the native extracellular matrix causes irreparable biomechanical changes and voice impairment. Here, in a series of in vitro and in vivo experiments, we identified differences in TGF-β1 and TGF-β3 transcription and immunolocalization to various cell subpopulations in naïve and injured rat VFM, compared with oral mucosa (which undergoes rapid healing with minimal scar) and skin (which typically heals with scar). Treatment of cultured human vocal fold fibroblasts with TGF-β3 resulted in less potent induction of profibrotic gene transcription, extracellular matrix synthesis and fibroblast-myofibroblast differentiation, compared with treatment with TGF-β1 and TGF-β2. Finally, delivery of exogenous TGF-β3 to rat VFM during the acute injury phase modulated the early inflammatory environment and reduced eventual scar formation. These experiments show that the TGF-β isoforms have distinct roles in VFM maintenance and repair, and that TGF-β3 redirects wound healing to improve VFM scar outcomes in vivo.
Keywords: Fibrosis; Larynx; Tissue repair; Wound healing.
Figures
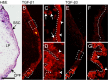
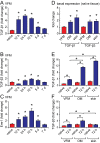
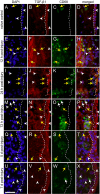
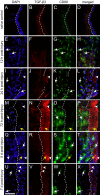
References
-
- Blakytny R., Ludlow A., Martin G. E. M., Ireland G., Lund L. R., Ferguson M. W. J., Brunner G. (2004). Latent TGF-β1 activation by platelets. J. Cell. Physiol. 199, 67–76 - PubMed
-
- Branski R. C., Verdolini K., Sandulache V., Rosen C. A., Hebda P. A. (2006). Vocal fold wound healing: a review for clinicians. J. Voice 20, 432–442 - PubMed
-
- Catten M., Gray S. D., Hammond T. H., Zhou R., Hammond E. (1998). Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngol. Head Neck Surg. 118, 663–667 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

