Inflammation, obesity, and thrombosis
- PMID: 24092932
- PMCID: PMC3829115
- DOI: 10.1182/blood-2013-05-427708
Inflammation, obesity, and thrombosis
Abstract
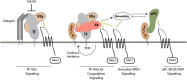
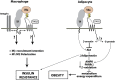
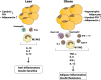
Clinical and epidemiological studies support a connection between obesity and thrombosis, involving elevated expression of the prothrombotic molecules plasminogen activator inhibitor-1 and tissue factor (TF) and increased platelet activation. Cardiovascular diseases and metabolic syndrome-associated disorders, including obesity, insulin resistance, type 2 diabetes, and hepatic steatosis, involve inflammation elicited by infiltration and activation of immune cells, particularly macrophages, into adipose tissue. Although TF has been clearly linked to a procoagulant state in obesity, emerging genetic and pharmacologic evidence indicate that TF signaling via G protein-coupled protease-activated receptors (PAR2, PAR1) additionally drives multiple aspects of the metabolic syndrome. TF-PAR2 signaling in adipocytes contributes to diet-induced obesity by decreasing metabolism and energy expenditure, whereas TF-PAR2 signaling in hematopoietic and myeloid cells drives adipose tissue inflammation, hepatic steatosis, and insulin resistance. TF-initiated coagulation leading to thrombin-PAR1 signaling also contributes to diet-induced hepatic steatosis and inflammation in certain models. Thus, in obese patients, clinical markers of a prothrombotic state may indicate a risk for the development of complications of the metabolic syndrome. Furthermore, TF-induced signaling could provide new therapeutic targets for drug development at the intersection between obesity, inflammation, and thrombosis.
Figures

References
-
- Morange PE, Alessi MC. Thrombosis in central obesity and metabolic syndrome: Mechanisms and epidemiology. Thromb Haemost. 2013;110(4):669–680. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. - PubMed
-
- Coutinho T, Goel K, Corrêa de Sá D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57(19):1877–1886. - PubMed
-
- Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. - PubMed
-
- Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118(9):978–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

