Distinct subcellular patterns of neprilysin protein and activity in the brains of Alzheimer's disease patients, transgenic mice and cultured human neuronal cells
- PMID: 24093058
- PMCID: PMC3786268
Distinct subcellular patterns of neprilysin protein and activity in the brains of Alzheimer's disease patients, transgenic mice and cultured human neuronal cells
Abstract
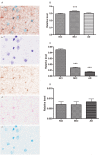
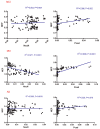
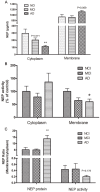
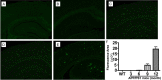
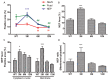
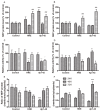
We investigated the subcellular distribution of NEP protein and activity in brains of human individuals with no cognitive impairment (NCI), mild cognitive impairment (MCI) and AD dementia, as well as double transgenic mice and human neuronal cell line treated with Aβ and 4-hydroxy-2-nonenal (HNE). Total cortical neuronal-related NEP was significantly increased in MCI compared to NCI brains. NeuN was decreased in both MCI and AD, consistent with neuronal loss occurring in MCI and AD. Negative relationship between NEP protein and NeuN in MCI brains, and positive correlation between NEP and pan-cadherin in NCI and MCI brains, suggesting the increased NEP expression in NCI and MCI might be due to membrane associated NEP in non-neuronal cells. In subcellular extracts, NEP protein decreased in cytoplasmic fractions in MCI and AD, but increased in membrane fractions, with a significant increase in the membrane/cytoplasmic ratio of NEP protein in AD brains. By contrast, NEP activity was decreased in AD. Similar results were observed in AD-mimic transgenic mice. Studies of SH-SY5Y neuroblastoma showed an up-regulation of NEP protein in the cytoplasmic compartment induced by HNE and Aβ; however, NEP activity decreased in cytoplasmic fractions. Activity of NEP in membrane fractions increased at 48 hours and then significantly decreased after treatment with HNE and Aβ. The cytoplasmic/membrane ratio of NEP protein increased at 24 hours and then decreased in both HNE and Aβ treated cells. Both HNE and Aβ up-regulate NEP expression, but NEP enzyme activity did not show the same increase, possibly indicating immature cytoplasmic NEP is less active than membrane associated NEP. These observations indicate that modulation of NEP protein levels and its subcellular location influence the net proteolytic activity and this complex association might participate in deficiency of Aβ degradation that is associated with amyloid deposition in AD.
Keywords: Alzheimer’s disease; Aβ clearance; Aβ degrading enzymes; amyloid-β; neprilysin; subcellular compartments.
Figures
Similar articles
-
Effects of HNE-modification induced by Abeta on neprilysin expression and activity in SH-SY5Y cells.J Neurochem. 2009 Feb;108(4):1072-82. doi: 10.1111/j.1471-4159.2008.05855.x. J Neurochem. 2009. PMID: 19196432 Free PMC article.
-
Oxidized neprilysin in aging and Alzheimer's disease brains.Biochem Biophys Res Commun. 2003 Oct 10;310(1):236-41. doi: 10.1016/j.bbrc.2003.09.003. Biochem Biophys Res Commun. 2003. PMID: 14511676
-
Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease.J Alzheimers Dis. 2015;44(4):1291-302. doi: 10.3233/JAD-142463. J Alzheimers Dis. 2015. PMID: 25408216
-
Impact of Insulin Degrading Enzyme and Neprilysin in Alzheimer's Disease Biology: Characterization of Putative Cognates for Therapeutic Applications.J Alzheimers Dis. 2015;48(4):891-917. doi: 10.3233/JAD-150379. J Alzheimers Dis. 2015. PMID: 26444774 Review.
-
Neprilysin and Aβ Clearance: Impact of the APP Intracellular Domain in NEP Regulation and Implications in Alzheimer's Disease.Front Aging Neurosci. 2013 Dec 23;5:98. doi: 10.3389/fnagi.2013.00098. Front Aging Neurosci. 2013. PMID: 24391587 Free PMC article. Review.
Cited by
-
Herpes simplex virus type 1 infection in neurons leads to production and nuclear localization of APP intracellular domain (AICD): implications for Alzheimer's disease pathogenesis.J Neurovirol. 2015 Oct;21(5):480-90. doi: 10.1007/s13365-015-0344-0. Epub 2015 Apr 30. J Neurovirol. 2015. PMID: 25925093
-
Religious Orders Study and Rush Memory and Aging Project.J Alzheimers Dis. 2018;64(s1):S161-S189. doi: 10.3233/JAD-179939. J Alzheimers Dis. 2018. PMID: 29865057 Free PMC article. Review.
-
Drosophila Neprilysin 1 Rescues Memory Deficits Caused by Amyloid-β Peptide.J Neurosci. 2017 Oct 25;37(43):10334-10345. doi: 10.1523/JNEUROSCI.1634-17.2017. Epub 2017 Sep 20. J Neurosci. 2017. PMID: 28931572 Free PMC article.
-
Somatostatin: Linking Cognition and Alzheimer Disease to Therapeutic Targeting.Pharmacol Rev. 2024 Oct 16;76(6):1291-1325. doi: 10.1124/pharmrev.124.001117. Pharmacol Rev. 2024. PMID: 39013601 Free PMC article. Review.
-
Sarsasapogenin-AA13 ameliorates Aβ-induced cognitive deficits via improving neuroglial capacity on Aβ clearance and antiinflammation.CNS Neurosci Ther. 2017 Jun;23(6):498-509. doi: 10.1111/cns.12697. Epub 2017 May 3. CNS Neurosci Ther. 2017. PMID: 28466999 Free PMC article.
References
-
- Selkoe DJ. Aging, amyloid, and Alzheimer’s disease: A perspective in honor of Carl Cotman. Neurochem Res. 2003;28:1705–1713. - PubMed
-
- Brown J, Smith S, Brun A, Collinge J, Gydesen S, Hardy J, Mullan M, Goate A. Genetic characterization of a novel familial dementia. Ann N Y Acad Sci. 1991;640:181–183. - PubMed
-
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. - PubMed
-
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. - PubMed
-
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu YH, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
