Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis
- PMID: 24093477
- PMCID: PMC4014806
- DOI: 10.1186/1465-9921-14-100
Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis
Abstract
Background: Clinicians are faced with an increasingly difficult choice regarding the optimal bronchodilator for patients with chronic obstructive pulmonary disease (COPD) given the number of new treatments. The objective of this study is to evaluate the comparative efficacy of indacaterol 75/150/300 μg once daily (OD), glycopyrronium bromide 50 μg OD, tiotropium bromide 18 μg/5 μg OD, salmeterol 50 μg twice daily (BID), formoterol 12 μg BID, and placebo for moderate to severe COPD.
Methods: Forty randomized controlled trials were combined in a Bayesian network meta-analysis. Outcomes of interest were trough and post-dose forced expiratory volume in 1 second (FEV1), St. George's Respiratory Questionnaire (SGRQ) score and responders (≥4 points), and Transition Dyspnea Index (TDI) score and responders (≥1 point) at 6 months.
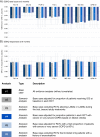
Results: Indacaterol was associated with a higher trough FEV1 than other active treatments (difference for indacaterol 150 μg and 300 μg versus placebo: 152 mL (95% credible interval (CrI): 126, 179); 160 mL (95% CrI: 133, 187)) and the greatest improvement in SGRQ score (difference for indacaterol 150 μg and 300 μg versus placebo: -3.9 (95% CrI -5.2, -2.6); -3.6 (95% CrI -4.8, -2.3)). Glycopyrronium and tiotropium 18 μg resulted in the next best estimates for both outcomes with minor differences (difference for glycopyrronium versus tiotropium for trough FEV1 and SGRQ: 18 mL (95% CrI: -16, 51); -0.55 (95% CrI: -2.04, 0.92).
Conclusion: In terms of trough FEV1 and SGRQ score indacaterol, glycopyrronium, and tiotropium are expected to be the most effective bronchodilators.
Figures





References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD): global strategy for the diagnosis management and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013Feb13.pdf.
-
- Firth R, Henley M, Kramer B, Lassen C, Yang W, Owen R. Full clinical study report for study number QAB149B2333: a phase III, 26-week multi-center randomized double-blind, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (150 and 300 μg od) in patients with chronic obstructive pulmonary disease. Novartis data on file. 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

