Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet
- PMID: 24093677
- PMCID: PMC3832130
- DOI: 10.1016/j.cmet.2013.09.004
Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet
Abstract
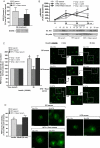
Obesity is associated with a chronic, low-grade, systemic inflammation that may contribute to the development of insulin resistance and type 2 diabetes. Resveratrol, a natural compound with anti-inflammatory properties, is shown to improve glucose tolerance and insulin sensitivity in obese mice and humans. Here, we tested the effect of a 2-year resveratrol administration on proinflammatory profile and insulin resistance caused by a high-fat, high-sugar (HFS) diet in white adipose tissue (WAT) from rhesus monkeys. Resveratrol supplementation (80 and 480 mg/day for the first and second year, respectively) decreased adipocyte size, increased sirtuin 1 expression, decreased NF-κB activation, and improved insulin sensitivity in visceral, but not subcutaneous, WAT from HFS-fed animals. These effects were reproduced in 3T3-L1 adipocytes cultured in media supplemented with serum from monkeys fed HFS ± resveratrol diets. In conclusion, chronic administration of resveratrol exerts beneficial metabolic and inflammatory adaptations in visceral WAT from diet-induced obese monkeys.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

