AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation
- PMID: 24093679
- PMCID: PMC3791399
- DOI: 10.1016/j.cmet.2013.08.019
AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation
Abstract
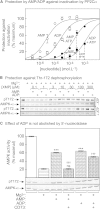
While allosteric activation of AMPK is triggered only by AMP, binding of both ADP and AMP has been reported to promote phosphorylation and inhibit dephosphorylation at Thr172. Because cellular concentrations of ADP and ATP are higher than AMP, it has been proposed that ADP is the physiological signal that promotes phosphorylation and that allosteric activation is not significant in vivo. However, we report that: AMP is 10-fold more potent than ADP in inhibiting Thr172 dephosphorylation; only AMP enhances LKB1-induced Thr172 phosphorylation; and AMP can cause > 10-fold allosteric activation even at concentrations 1-2 orders of magnitude lower than ATP. We also provide evidence that allosteric activation by AMP can cause increased phosphorylation of acetyl-CoA carboxylase in intact cells under conditions in which there is no change in Thr172 phosphorylation. Thus, AMP is a true physiological regulator of AMPK, and allosteric regulation is an important component of the overall activation mechanism.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Amodeo G.A., Rudolph M.J., Tong L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 2007;449:492–495. - PubMed
-
- Carling D., Zammit V.A., Hardie D.G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. - PubMed
-
- Carling D., Clarke P.R., Zammit V.A., Hardie D.G. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur. J. Biochem. 1989;186:129–136. - PubMed
-
- Carling D., Thornton C., Woods A., Sanders M.J. AMP-activated protein kinase: new regulation, new roles? Biochem. J. 2012;445:11–27. - PubMed
-
- Chen L., Wang J., Zhang Y.Y., Yan S.F., Neumann D., Schlattner U., Wang Z.X., Wu J.W. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat. Struct. Mol. Biol. 2012;19:716–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

