Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP
- PMID: 24093681
- PMCID: PMC3822903
- DOI: 10.1016/j.cmet.2013.09.009
Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP
Abstract
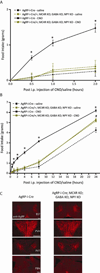
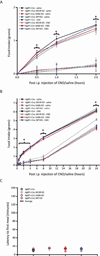
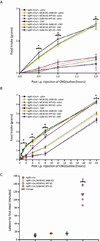
Agouti-related peptide (AgRP) neurons of the hypothalamus release a fast transmitter (GABA) in addition to neuropeptides (neuropeptide Y [NPY] and Agouti-related peptide [AgRP]). This raises questions as to their respective functions. The acute activation of AgRP neurons robustly promotes food intake, while central injections of AgRP, NPY, or GABA agonist results in the marked escalation of food consumption with temporal variance. Given the orexigenic capability of all three of these neuroactive substances in conjunction with their coexpression in AgRP neurons, we looked to unravel their relative temporal role in driving food intake. After the acute stimulation of AgRP neurons with DREADD technology, we found that either GABA or NPY is required for the rapid stimulation of feeding, and the neuropeptide AgRP, through action on MC4 receptors, is sufficient to induce feeding over a delayed yet prolonged period. These studies help to elucidate the neurochemical mechanisms of AgRP neurons in controlling temporally distinct phases of eating.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

