Transmembrane current imaging in the heart during pacing and fibrillation
- PMID: 24094412
- PMCID: PMC3791310
- DOI: 10.1016/j.bpj.2013.08.019
Transmembrane current imaging in the heart during pacing and fibrillation
Abstract
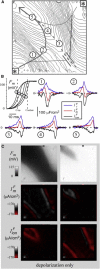
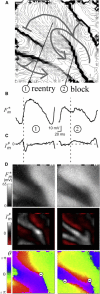
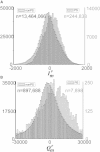
Recently, we described a method to quantify the time course of total transmembrane current (Im) and the relative role of its two components, a capacitive current (Ic) and a resistive current (Iion), corresponding to the cardiac action potential during stable propagation. That approach involved recording high-fidelity (200 kHz) transmembrane potential (Vm) signals with glass microelectrodes at one site using a spatiotemporal coordinate transformation via measured conduction velocity. Here we extend our method to compute these transmembrane currents during stable and unstable propagation from fluorescence signals of Vm at thousands of sites (3 kHz), thereby introducing transmembrane current imaging. In contrast to commonly used linear Laplacians of extracellular potential (Ve) to compute Im, we utilized nonlinear image processing to compute the required second spatial derivatives of Vm. We quantified the dynamic spatial patterns of current density of Im and Iion for both depolarization and repolarization during pacing (including nonplanar patterns) by calibrating data with the microelectrode signals. Compared to planar propagation, we found that the magnitude of Iion was significantly reduced at sites of wave collision during depolarization but not repolarization. Finally, we present uncalibrated dynamic patterns of Im during ventricular fibrillation and show that Im at singularity sites was monophasic and positive with a significant nonzero charge (Im integrated over 10 ms) in contrast with nonsingularity sites. Our approach should greatly enhance the understanding of the relative roles of functional (e.g., rate-dependent membrane dynamics and propagation patterns) and static spatial heterogeneities (e.g., spatial differences in tissue resistance) via recordings during normal and compromised propagation, including arrhythmias.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Cabo C., Pertsov A.M., Jalife J. Wave-front curvature as a cause of slow conduction and block in isolated cardiac muscle. Circ. Res. 1994;75:1014–1028. - PubMed
-
- Gray R.A., Jalife J., Pertsov A.M. Nonstationary vortexlike reentrant activity as a mechanism of polymorphic ventricular tachycardia in the isolated rabbit heart. Circulation. 1995;91:2454–2469. - PubMed
-
- Gray R.A., Jalife J., Pertsov A.M. Mechanisms of cardiac fibrillation. Science. 1995;270:1222–1223. - PubMed
-
- Gray R.A., Pertsov A.M., Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

