The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch
- PMID: 24094650
- PMCID: PMC4041105
- DOI: 10.1016/j.cell.2013.08.057
The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch
Abstract
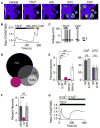
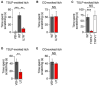
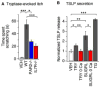
Atopic dermatitis (AD) is a chronic itch and inflammatory disorder of the skin that affects one in ten people. Patients suffering from severe AD eventually progress to develop asthma and allergic rhinitis, in a process known as the "atopic march." Signaling between epithelial cells and innate immune cells via the cytokine thymic stromal lymphopoietin (TSLP) is thought to drive AD and the atopic march. Here, we report that epithelial cells directly communicate to cutaneous sensory neurons via TSLP to promote itch. We identify the ORAI1/NFAT calcium signaling pathway as an essential regulator of TSLP release from keratinocytes, the primary epithelial cells of the skin. TSLP then acts directly on a subset of TRPA1-positive sensory neurons to trigger robust itch behaviors. Our results support a model whereby calcium-dependent TSLP release by keratinocytes activates both primary afferent neurons and immune cells to promote inflammatory responses in the skin and airways.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Comment in
-
The missing link between itch and inflammation in atopic dermatitis.Cell. 2013 Oct 10;155(2):267-9. doi: 10.1016/j.cell.2013.09.038. Cell. 2013. PMID: 24120126 Free PMC article.
-
A new itch to scratch for TSLP.Trends Immunol. 2014 Feb;35(2):49-50. doi: 10.1016/j.it.2013.12.001. Epub 2014 Jan 9. Trends Immunol. 2014. PMID: 24412411 Free PMC article.
References
-
- Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. - PubMed
-
- Almers N. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Letters. 1985;192:13–18. - PubMed
-
- Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. J Invest Dermatol. 2001;117:1621–1626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

