53BP1: pro choice in DNA repair
- PMID: 24094932
- PMCID: PMC3946699
- DOI: 10.1016/j.tcb.2013.09.003
53BP1: pro choice in DNA repair
Abstract
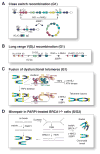
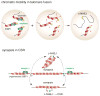
The DNA damage response factor 53BP1 functions at the intersection of two major double strand break (DSB) repair pathways--promoting nonhomologous end-joining (NHEJ) and inhibiting homology-directed repair (HDR)--and integrates cellular inputs to ensure their timely execution in the proper cellular contexts. Recent work has revealed that 53BP1 controls 5' end resection at DNA ends, mediates synapsis of DNA ends, promotes the mobility of damaged chromatin, improves DSB repair in heterochromatic regions, and contributes to lethal mis-repair of DSBs in BRCA1-deficient cells. Here we review these aspects of 53BP1 and discuss new data revealing how 53BP1 is loaded onto chromatin and uses its interacting factors Rif1 and PTIP to promote NHEJ and inhibit HDR.
Keywords: 53BP1; BRCA1; CSR; HDR; NHEJ; PARPi; PTIP; Rif1; V(D)J; resection; telomere.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Noon AT, Goodarzi AA. 53BP1-mediated DNA double strand break repair: insert bad pun here. DNA Repair (Amst) 2011;10:1071–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

