Vein graft failure
- PMID: 24095042
- PMCID: PMC4391818
- DOI: 10.1016/j.jvs.2013.08.019
Vein graft failure
Abstract
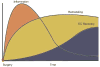
After the creation of an autogenous lower extremity bypass graft, the vein must undergo a series of dynamic structural changes to stabilize the arterial hemodynamic forces. These changes, which are commonly referred to as remodeling, include an inflammatory response, the development of a neointima, matrix turnover, and cellular proliferation and apoptosis. The sum total of these processes results in dramatic alterations in the physical and biomechanical attributes of the arterialized vein. The most clinically obvious and easily measured of these is lumen remodeling of the graft. However, although somewhat less precise, wall thickness, matrix composition, and endothelial changes can be measured in vivo within the healing vein graft. Recent translational work has demonstrated the clinical relevance of remodeling as it relates to vein graft patency and the systemic factors influencing it. By correlating histologic and molecular changes in the vein, insights into potential therapeutic strategies to prevent bypass failure and areas for future investigation are explored.
Copyright © 2015 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author conflict of interest: none
Figures



References
-
- Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. Jama. 2005;294(19):2446–54. - PubMed
-
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. discussion 751. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

