Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: repair of the extracellular matrix
- PMID: 24095195
- PMCID: PMC3959900
- DOI: 10.1016/S0140-6736(13)61897-8
Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: repair of the extracellular matrix
Abstract
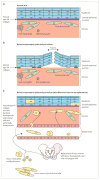
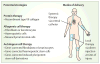
Contrary to the prevailing professional opinion of the past few decades, recent experimental and clinical data support the fact that protein replacement therapy by allogeneic blood and marrow transplantation is not limited to freely diffusible molecules such as enzymes, but also large structural proteins such as collagens. A prime example is the cross-correction of type VII collagen deficiency in generalised severe recessive dystrophic epidermolysis bullosa, in which blood and marrow transplantation can attenuate the mucocutaneous manifestations of the disease and improve patients' quality of life. Although allogeneic blood and marrow transplantation can improve the integrity of the skin and mucous membranes, today's accomplishments are only the first steps on the long pathway to cure. Future strategies will be built on the lessons learned from these first transplant studies.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures





References
-
- Bruckner-Tuderman L. Dystrophic epidermolysis bullosa: pathogenesis and clinical features. Dermatol Clin. 2010;28:107–14. - PubMed
-
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931–50. - PubMed
-
- Uitto J, McGrath JA, Rodeck U, Bruckner-Tuderman L, Robinson EC. Progress in epidermolysis bullosa research: toward treatment and cure. J Invest Dermatol. 2010;130:1778–84. - PubMed
-
- Mellerio JE. Infection and colonization in epidermolysis bullosa. Dermatol Clin. 2010;28:267–69. - PubMed
-
- Pope E, Lara-Corrales I, Mellerio JE, Martinez AE, Sibbald C, Sibbald RG. Epidermolysis bullosa and chronic wounds: a model for wound bed preparation of fragile skin. Adv Skin Wound Care. 2013;26:177–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

