The antiviral activities of ISG15
- PMID: 24095857
- PMCID: PMC4090058
- DOI: 10.1016/j.jmb.2013.09.041
The antiviral activities of ISG15
Abstract
Post-translational protein modification is an important strategy for the regulation of the cell proteome independent of the need for new gene expression. Ubiquitin and ubiquitin-like modifiers mediate the regulation of protein levels, signaling pathways, vesicular trafficking, and many other cellular processes through their covalent conjugation to proteins. Interferon stimulated gene 15 (ISG15) is a ubiquitin-like modifier induced by type I interferon. In addition to conjugating to potentially hundreds of target proteins, ISG15 can be found in an unconjugated form both inside of the cell and released from interferon stimulated cells into the extracellular environment. Due to its robust expression after type I interferon stimulation and the broad panel of proteins that it targets, ISG15 has drawn much attention as a potential regulator of the immune response and has been shown to mediate protection in a number of different viral infection models. Here we will review the current state of the field of ISG15, the viruses against which ISG15 mediates protection, and the mechanisms by which ISG15 exerts antiviral activity.
Keywords: ASLV; CHIKV; Chikungunya virus; ESCRT; HIV-1; HPV; HSV-1; IRF3; ISG15; JNK; Jun N-terminal kinase; LCMV; MEF; NDV; NK; Newcastle disease virus; PKR; RIG-I; SeV; Sendai virus; VLP; VSV; WNV; WT; West Nile virus; avian sarcoma leukosis virus; endosomal sorting complexes required for transport; herpes simplex virus 1; human immunodeficiency virus 1; human papilloma virus; innate immunity; interferon regulatory factor 3; interferon stimulated gene 15; interferons; lymphocytic choriomeningitis virus; mouse embryonic fibroblast; natural killer; protein kinase R; retinoic acid inducible gene 1; short interfering RNA; siRNA; ubiquitin-like protein; vesicular stomatitis virus; virus-like particle; viruses; wild type.
© 2013.
Figures

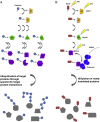
References
-
- Hershko A., Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Ciechanover A. Tracing the history of the ubiquitin proteolytic system: the pioneering article. Biochem Biophys Res Commun. 2009;387:1–10. - PubMed
-
- Komander D., Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. - PubMed
-
- van der Veen A.G., Ploegh H.L. Ubiquitin-like proteins. Annu Rev Biochem. 2012;81:323–357. - PubMed
-
- Korant B.D., Blomstrom D.C., Jonak G.J., Knight E. Interferon-induced proteins. Purification and characterization of a 15,000-dalton protein from human and bovine cells induced by interferon. J Biol Chem. 1984;259:14835–14839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

