DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer
- PMID: 24096005
- PMCID: PMC3910427
- DOI: 10.1053/j.gastro.2013.09.050
DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer
Abstract
Background & aims: As in other tumor types, progression of pancreatic cancer may require a functionally unique population of cancer stem cells. Although such cells have been identified in many invasive cancers, it is not clear whether they emerge during early or late stages of tumorigenesis. Using mouse models and human pancreatic cancer cell lines, we investigated whether preinvasive pancreatic neoplasia contains a subpopulation of cells with distinct morphologies and cancer stem cell-like properties.
Methods: Pancreatic tissue samples were collected from the KC(Pdx1), KPC(Pdx1), and KC(iMist1) mouse models of pancreatic intraepithelial neoplasia (PanIN) and analyzed by confocal and electron microscopy, lineage tracing, and fluorescence-activated cell sorting. Subpopulations of human pancreatic ductal adenocarcinoma (PDAC) cells were similarly analyzed and also used in complementary DNA microarray analyses.
Results: The microtubule regulator DCLK1 marked a morphologically distinct and functionally unique population of pancreatic cancer-initiating cells. These cells displayed morphological and molecular features of gastrointestinal tuft cells. Cells that expressed DCLK1 also expressed high levels of ATAT1, HES1, HEY1, IGF1R, and ABL1, and manipulation of these pathways in PDAC cell lines inhibited their clonogenic potential. Pharmacological inhibition of γ-secretase activity reduced the abundance of these cells in murine PanIN in a manner that correlated with inhibition of PanIN progression.
Conclusions: Human PDAC cells and pancreatic neoplasms in mice contain morphologically and functionally distinct subpopulations that have cancer stem cell-like properties. These populations can be identified at the earliest stages of pancreatic tumorigenesis and provide new cellular and molecular targets for pancreatic cancer treatment and/or chemoprevention.
Keywords: ADM; AcTub; Acetylated Tubulin; Dclk1; FACS; GFP; Kras; Notch; PDAC; PanIN; TEM; acetylated α-tubulin; acinar-to-ductal metaplasia; doublecortin and Ca(2+)/calmodulin-dependent kinase-like 1; fluorescence-activated cell sorting; green fluorescent protein; mPanIN; murine pancreatic intraepithelial neoplasia; pancreatic ductal adenocarcinoma; pancreatic intraepithelial neoplasia; transmission electron microscopy.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Jennifer M. Bailey has no Conflict of interest
Janivett Alsina has no Conflict of Interest
Zeshaan A. Rasheed has no Conflict of Interest
Ya-Yuan Fu has no Conflict of Interest
Ruben Pletz has no conflict of Interest
Florencia McAllister has no Conflict of Interest
Hao Zhang has no Conflict of Interest
Nabeel Bardeesy has no conflict of interest
Pankaj Pasricha has no Conflict of Interest
William Matsui has no Conflict of Interest
Anirban Maitra has no Conflict of Interest
Steven Leach has no Conflict of Interest
Figures
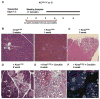
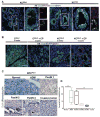



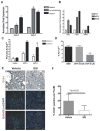
Comment in
-
New insights into the cell lineage of pancreatic ductal adenocarcinoma: evidence for tumor stem cells in premalignant lesions?Gastroenterology. 2014 Jan;146(1):24-6. doi: 10.1053/j.gastro.2013.11.023. Epub 2013 Nov 22. Gastroenterology. 2014. PMID: 24275238 No abstract available.
-
Insights into cell lineage of pancreatic adenocarcinoma.Gastroenterology. 2014 Jan;146(1):3-4. Gastroenterology. 2014. PMID: 24501750 No abstract available.
References
-
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. - PubMed
-
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. - PubMed
-
- Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, Goggins M, Iacobuzio-Donahue C, Berman DM, Laheru D, Jimeno A, Hidalgo M, Maitra A, Matsui W. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

