Theranostic immunoliposomes for osteoarthritis
- PMID: 24096032
- PMCID: PMC4962614
- DOI: 10.1016/j.nano.2013.09.004
Theranostic immunoliposomes for osteoarthritis
Abstract
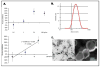
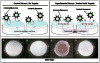
Although there have been substantial advancements in the treatment of inflammatory arthritis, treatments for osteoarthritis (OA) have lagged and currently are primarily palliative until joints become totally dysfunctional and prosthetic replacement is needed. One obstacle for developing a preventive therapy for OA is the lack of good tools for efficiently diagnosing the disease and monitoring its progression during the early stages when the effect of therapeutic drugs or biologics is most likely to be effective. We have developed near infrared immunoliposomes conjugated with type II collagen antibody for diagnosis and treatment of early OA. These immunoliposomes bind to damaged but not normal cartilage. Utilizing these reagents, we can quantitate exposure of type II collagen during cartilage degradation in individual joints in vivo in a guinea pig. Immunoliposomes could be used to determine the effectiveness of therapeutic interventions in small animals as well as vehicles for localized drug delivery to OA chondrocytes.
From the clinical editor: This team of authors have developed near infrared immunoliposomes conjugated with type II collagen antibody for diagnosis and treatment of early OA, with promising results demonstrated in a guinea pig model.
Keywords: Extracellular matrix (ECM); Immuno-liposomes (ILs); Near-infrared (NIR) fluorescent; Osteoarthritis (OA); Theranostic (therapeutic and diagnostic); Type II collagen (CII).
© 2014.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures


References
-
- Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology. 2008;47:1452–1460. - PubMed
-
- Bitton R. The economic burden of Osteoarthritis. Am J Manag Care. 2009;15:230–235. - PubMed
-
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis; An untreatable disease? Nature Review. 2005;4:331–344. - PubMed
-
- Elsaid KA, Chichester CO. Collagen markers in early arthritic diseases. Clinica Chimica Acta. 2006;365:68–77. - PubMed
-
- Jasin HE, Noyori K, Takagi T, Taurog JD. Characteristics of anti-type II collagen antibody binding to articular cartilage. Arthritis Rheum. 1993;36(5):651–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

