Kinetic framework of spindle assembly checkpoint signalling
- PMID: 24096243
- PMCID: PMC4067996
- DOI: 10.1038/ncb2842
Kinetic framework of spindle assembly checkpoint signalling
Abstract
The mitotic spindle assembly checkpoint (SAC) delays anaphase onset until all chromosomes have attached to both spindle poles. Here, we investigated SAC signalling kinetics in response to acute detachment of individual chromosomes using laser microsurgery. Most detached chromosomes delayed anaphase until they had realigned to the metaphase plate. A substantial fraction of cells, however, entered anaphase in the presence of unaligned chromosomes. We identify two mechanisms by which cells can bypass the SAC: first, single unattached chromosomes inhibit the anaphase-promoting complex/cyclosome (APC/C) less efficiently than a full complement of unattached chromosomes; second, because of the relatively slow kinetics of re-imposing APC/C inhibition during metaphase, cells were unresponsive to chromosome detachment up to several minutes before anaphase onset. Our study defines when cells irreversibly commit to enter anaphase and shows that the SAC signal strength correlates with the number of unattached chromosomes. Detailed knowledge about SAC signalling kinetics is important for understanding the emergence of aneuploidy and the response of cancer cells to chemotherapeutics targeting the mitotic spindle.
Figures
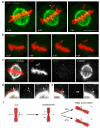
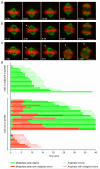

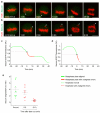
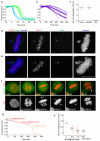
Comment in
-
Slipping past the spindle assembly checkpoint.Nat Cell Biol. 2013 Nov;15(11):1261-3. doi: 10.1038/ncb2876. Nat Cell Biol. 2013. PMID: 24189515
References
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
-
- Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–980. - PubMed
-
- Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–581. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

