Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study
- PMID: 24096620
- PMCID: PMC3814411
- DOI: 10.1093/jnci/djt235
Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study
Abstract
Background: Pap cytology is known to be more specific but less sensitive than testing for human papillomavirus (HPV) for the detection of high-grade cervical intraepithelial neoplasia (CIN2+). We assessed whether p16/Ki-67 dual-stained cytology, a biomarker combination indicative of transforming HPV infections, can provide high sensitivity for CIN2+ in screening while maintaining high specificity. Results were compared with Pap cytology and HPV testing.
Methods: A total of 27,349 women 18 years or older attending routine cervical cancer screening were prospectively enrolled in five European countries. Pap cytology, p16/Ki-67 immunostaining, and HPV testing were performed on all women. Positive test results triggered colposcopy referral, except for women younger than 30 years with only positive HPV test results. Presence of CIN2+ on adjudicated histology was used as the reference standard. Two-sided bias-corrected McNemar P values were determined.
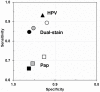
Results: The p16/Ki-67 dual-stained cytology positivity rates were comparable with the prevalence of abnormal Pap cytology results and less than 50% of the positivity rates observed for HPV testing. In women of all ages, dual-stained cytology was more sensitive than Pap cytology (86.7% vs 68.5%; P < .001) for detecting CIN2+, with comparable specificity (95.2% vs 95.4%; P = .15). The relative performance of the tests was similar in both groups of women: younger than age 30 and 30 years or older. HPV testing in women 30 years or older was more sensitive than dual-stained cytology (93.3% vs 84.7%; P = .03) but less specific (93.0% vs 96.2%; P < .001).
Conclusions: The p16/Ki-67 dual-stained cytology combines superior sensitivity and noninferior specificity over Pap cytology for detecting CIN2+. It suggests a potential role of dual-stained cytology in screening, especially in younger women where HPV testing has its limitations.
Figures
Comment in
-
From Papanicolaou to papillomaviruses: evolving challenges in cervical cancer screening in the era of human papillomavirus vaccination.J Natl Cancer Inst. 2013 Oct 16;105(20):1524-6. doi: 10.1093/jnci/djt267. Epub 2013 Oct 4. J Natl Cancer Inst. 2013. PMID: 24096622 Free PMC article. No abstract available.
References
-
- Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–819 - PubMed
-
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–F99 - PubMed
-
- Castle PE, Stoler MH, Wright TC, Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12(9):880–890 - PubMed
-
- Bulkman NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007;370(9601):1764–1772 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

