Run-in phase III trial design with pharmacodynamics predictive biomarkers
- PMID: 24096624
- PMCID: PMC3888165
- DOI: 10.1093/jnci/djt265
Run-in phase III trial design with pharmacodynamics predictive biomarkers
Abstract
Background: Developments in biotechnology have stimulated the use of predictive biomarkers to identify patients who are likely to benefit from a targeted therapy. Several randomized phase III designs have been introduced for development of a targeted therapy using a diagnostic test. Most such designs require biomarkers measured before treatment. In many cases, it has been very difficult to identify such biomarkers. Promising candidate biomarkers can sometimes be effectively measured after a short run-in period on the new treatment.
Methods: We introduce a new design for phase III trials with a candidate predictive pharmacodynamic biomarker measured after a short run-in period. Depending on the therapy and the biomarker performance, the trial would either randomize all patients but perform a separate analysis on the biomarker-positive patients or only randomize marker-positive patients after the run-in period. We evaluate the proposed design compared with the conventional phase III design and discuss how to design a run-in trial based on phase II studies.
Results: The proposed design achieves a major sample size reduction compared with the conventional randomized phase III design in many cases when the biomarker has good sensitivity (≥0.7) and specificity (≥0.7). This requires that the biomarker be measured accurately and be indicative of drug activity. However, the proposed design loses some of its advantage when the proportion of potential responders is large (>50%) or the effect on survival from run-in period is substantial.
Conclusions: Incorporating a pharmacodynamic biomarker requires careful consideration but can expand the capacity of clinical trials to personalize treatment decisions and enhance therapeutics development.
Figures
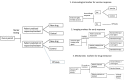

 = 1).
= 1).
 = 1). The standard design needs 1440, 420, and 210 events to have 80% power under 25%, 50%, and 75% true responders, respectively.
= 1). The standard design needs 1440, 420, and 210 events to have 80% power under 25%, 50%, and 75% true responders, respectively.References
-
- Sargent DJ, Conley BA, Allegra C, et al. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23(9):2020–2027 - PubMed
-
- Maitournam A, Simon R. On the efficiency of targeted clinical trials. Stat Med. 2005;24(3):329–339 - PubMed
-
- Simon R, Maitournam A. Evaluating the efficiency of targeted designs for randomized clinical trials. Clin Cancer Res. 2004;10(20):6759–6763 - PubMed
-
- Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–788 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

