Transdermal fentanyl for cancer pain
- PMID: 24096644
- PMCID: PMC6517042
- DOI: 10.1002/14651858.CD010270.pub2
Transdermal fentanyl for cancer pain
Abstract
Background: Opioid drugs have been used for many years to relieve pain. Transdermal fentanyl offers one option for delivering and maintaining pain relief in patients with moderate or severe cancer pain.
Objectives: To determine the analgesic efficacy of transdermal fentanyl for relief of cancer pain, and to assess the adverse events associated with the use of transdermal fentanyl for relief of cancer pain.
Search methods: The following databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4 of 12); MEDLINE (1966 to May 2013); EMBASE (1974 to May 2013; CANCERLIT (PubMED) (November 2012); and ClinicalTrials.gov (May 2013).
Selection criteria: Published randomised controlled trials (RCTs) using placebo or active comparators reporting on the analgesic effect of transdermal fentanyl in adults and children with cancer pain. Studies with fewer than 10 participants were excluded.
Data collection and analysis: Data were extracted independently by two review authors. We extracted any available data on the number or proportion of patients with 'no worse than mild pain' or treatment success (very satisfied, or very good or excellent on patient global impression scales), together with information about adverse events and withdrawals.
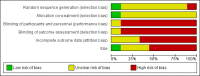
Main results: We identified nine studies meeting the inclusion criteria, including a Turkish study that is awaiting formal translation. There were 1244 participants randomised in classically designed RCTs, of whom 1197 had evaluable data, and 138 patients enrolled in an enriched enrolment, randomised withdrawal (EERW) trial. Overall, 600 participants were treated with transdermal fentanyl patches, 382 with various formulations of morphine, 36 with methadone, and 221 with paracetamol plus codeine. There were major sources of potential bias, including lack of blinding, small size, high levels of attrition, and inconsistent reporting.We could not compare data in a meaningful analysis regarding adverse events such as nausea, abdominal pain, gastrointestinal bleeding, and confusion. These events may have been attributable to the underlying disease process.There were insufficient comparable data for meta-analysis to be undertaken or to produce numbers needed to treat (NNT) for the analgesic effect. In seven studies with 461 participants reporting pain intensity results after about two weeks, the mean or median pain scores were on the borderline of mild and moderate pain. Most participants would have had no worse than mild pain on treatment. Another reported that 77% of participants using transdermal fentanyl had an undefined successful outcome. Fewer participants experienced constipation with transdermal fentanyl (28%) than with oral morphine (46%), giving a risk ratio of 0.61 (95% CI 0.47 to 0.78); the NNT to prevent constipation was 5.5 (95% CI 3.8 to 10).
Authors' conclusions: The randomised trial literature for effectiveness of transdermal fentanyl is limited, but it is an important medicine. Most studies recruited fewer than 100 participants and did not provide data appropriate for meta-analysis. Only a few reported how many patients had good pain relief but, where data were reported, a majority had no worse than mild pain within a reasonably short time period. The evidence pointed to a useful and significant reduction in complaints about constipation for transdermal fentanyl compared with oral morphine.
Conflict of interest statement
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. PW and GH have no relevant interests to declare.
Figures



Update of
References
References to studies included in this review
Ahmedzai 1997 {published data only}
Kongsgaard 1998 {published data only}
-
- Kongsgaard UE, Poulain P. Transdermal fentanyl for pain control in adults with chronic cancer pain. European Journal of Pain 1998;2(1):53‐62. - PubMed
Kress 2008 {published data only}
-
- Kress HG, Laage D, Hoerauf KH, Nolte T, Heiskanen T, Petersen R, et al. A randomized, open, parallel group, multicenter trial to investigate analgesic efficacy and safety of a new transdermal fentanyl patch compared to standard opioid treatment in cancer pain. Journal of Pain and Symptom Management 2008;36(3):268‐79. [DOI: 10.1016/j.jpainsymman.2007.10.023] - DOI - PubMed
Mercadante 2008 {published data only}
Mystakidou 2005 {published data only}
-
- Mystakidou K, Katsouda E, Kouloulias V, Kouvaris J, Tsiatas M, Vlahos L. Comparison of transdermal fentanyl with codeine/paracetamol, in combination with radiotherapy, for the management of metastatic bone pain. Journal of Opioid Management 2005;1(4):204‐10. - PubMed
Oztürk 2008 {published data only}
-
- Oztürk T, Karadibak K, Catal D, Cakan A, Tugsavul F, Cirak K. Comparison of TD‐fentanyl with sustained‐release morphine in the pain treatment of patients with lung cancer. Agri 2008;20(3):20‐5. - PubMed
Pistevou‐Gompaki 2004 {published data only}
-
- Pistevou‐Gompaki K, Kouloulias VE, Varveris C, Mystakidou K, Georgakopoulos G, Eleftheriadis N, et al. Radiotherapy plus either transdermal fentanyl or paracetamol and codeine for painful bone metastases: a randomised study of pain relief and quality of life. Current Medical Research and Opinion 2004;20(2):159‐63. [DOI: 10.1185/030079903125002829] - DOI - PubMed
van Seventer 2003 {published data only}
Wong 1997 {published data only}
-
- Wong JO, Chiu GL, Tsao CJ, Chang CL. Comparison of oral controlled‐release morphine with transdermal fentanyl in terminal cancer pain. Acta Anaesthesiologica Sinica 1997;35(3):25‐32. - PubMed
References to studies excluded from this review
Ergenoglu 2010 {published data only}
-
- Ergenoglu P, Ozbek H, Gunduz M, Isik G. Comparison of the analgesic efficacy of intravenous morphine infusion and transdermal fentanyl in oncology patients with mucositis [Mukozitli onkoloji hastalarinda intravenoz morfin infuzyonu ve transdermal fentanilin analjezik etkinliginin karsilastirilmasi]. Anatolian Journal of Clinical Investigation 2010;4(3):134‐40.
Sarhan 2009 {published data only}
-
- Sarhan T, Doghem M. A comparison of two trans‐dermal drug delivery systems; buprenorphine and fentanyl for chronic cancer pain management. European Journal of Pain. 6th Congress of the European Federation of IASP Chapters: Pain in Europe 6th, EFIC Lisbon Portugal. 2009; Vol. 13:S199.
Wirz 2009 {published data only}
-
- Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, et al. Gastrointestinal symptoms under opioid therapy: a prospective comparison of oral sustained‐release hydromorphone, transdermal fentanyl, and transdermal buprenorphine. European Journal of Pain 2009;13(7):737‐43. [DOI: 10.1016/j.ejpain.2008.09.005] - DOI - PubMed
Additional references
Cachia 2011
Cancer Research UK
-
- Cancer Research UK. Cancer incidence for all cancers combined. http://info.cancerresearchuk.org/cancerstats/incidence/all‐cancers‐combi... [Accessed 22 May 2013].
Clark 2004
-
- Clark AJ, Ahmedzai SH, Allan LG, Camacho F, Horbay GL, Richarz U, et al. Efficacy and safety of transdermal fentanyl and sustained‐release oral morphine in patients with cancer and chronic non‐cancer pain. Current Medical Research and Opinion 2004;20(9):1419‐28. [DOI: 10.1185/030079904X2114] - DOI - PubMed
Cleary 2007
Cook 1995
Dworkin 2008
Electronic medicines compendium
-
- Durogesic® DTrans. http://www.medicines.org.uk/EMC/medicine/17088/ (Accessed 22 May 2013).
Hadley 2009
Heiskanen 2009
Higgins 2011
-
- Altman DG, Antes G, Gøtzsche P, Higgins JPT, Jüni P, Lewis S, et al. Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Jadad 1996
Jeal 1997
-
- Jeal W, Benfield P. A review of its pharmacological properties and therapeutic efficacy in pain control. Drugs 1997;53(1):109‐38. - PubMed
Kornick 2003
-
- Kornick CA, Santiago‐Palma J, Moryl N, Payne R, Obbens EA. Benefit‐risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Safety 2003;26(13):951‐73. - PubMed
Koyyalagunta 2012
-
- Koyyalagunta D, Bruera E, Solanki DR, Nouri KH, Burton AW, Toro MP, et al. A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 2012;15(3 Suppl):ES39‐58. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
Lehmann 1992
-
- Lehmann KA, Zech D. Transdermal fentanyl: clinical pharmacology. Journal of Pain and Symptom Management 1992;7 3 Suppl:S8‐16. - PubMed
Marinangeli 2007
McQuay 1998
-
- McQuay H, Moore R. An evidence‐based resource for pain relief. An evidence‐based resource for pain relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
Moore 1982
-
- Moore RA, Bullingham RE, McQuay HJ, Hand CW, Aspel JB, Allen MC, et al. Dural permeability to narcotics: in vitro determination and application to extradural administration. British Journal of Anaesthetics 1982;54(10):1117‐28. - PubMed
Moore 1998
Moore 2010
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Morris 1995
Muijsers 2001
-
- Muijsers RB, Wagstaff AJ. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs 2001;61(15):2289‐307. - PubMed
RevMan 2011
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tassinari 2008
-
- Tassinari D, Sartori S, Tamburini E, Scarpi E, Raffaeli W, Tombesi P, et al. Adverse effects of transdermal opiates treating moderate‐severe cancer pain in comparison to long‐acting morphine: a meta‐analysis and systematic review of the literature. Journal of Palliative Medicine 2008;11(3):492‐501. [DOI: 10.1089/jpm.2007.0200] - DOI - PubMed
Tassinari 2011
Tramèr 1997
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

