Efficacy of anti-inflammatory or antibiotic treatment in patients with non-complicated acute bronchitis and discoloured sputum: randomised placebo controlled trial
- PMID: 24097128
- PMCID: PMC3790568
- DOI: 10.1136/bmj.f5762
Efficacy of anti-inflammatory or antibiotic treatment in patients with non-complicated acute bronchitis and discoloured sputum: randomised placebo controlled trial
Abstract
Objective: To evaluate the efficacy of oral anti-inflammatory or antibiotic treatment compared with placebo in the resolution of cough in patients with uncomplicated acute bronchitis and discoloured sputum.
Design: Multicentre, parallel, single blinded placebo controlled, randomised clinical trial.
Setting: Nine primary care centres in Spain.
Participants: Adults aged 18 to 70 presenting symptoms associated with respiratory tract infection of less than one week's duration, with cough as the predominant symptom, the presence of discoloured sputum, and at least one other symptom of lower respiratory tract infection (dyspnoea, wheezing, chest discomfort, or chest pain).
Interventions: Patients were randomised to receive either ibuprofen 600 mg three times daily, amoxicillin-clavulanic acid 500 mg/125 mg three times daily, or placebo three times daily for 10 days. The duration of symptoms was measured with a diary card.
Main outcome measure: Number of days with frequent cough after the randomisation visit.
Results: 416 participants were randomised (136 to ibuprofen, 137 to antibiotic, and 143 to placebo) and 390 returned their symptom diaries fully completed. The median number of days with frequent cough was slightly lower among patients assigned to ibuprofen (9 days, 95% confidence interval 8 to 10 days) compared with those receiving amoxicillin-clavulanic acid (11 days, 10 to 12 days) or placebo (11 days, 8 to 14 days), albeit without statistically significant differences. Neither amoxicillin-clavulanic acid nor ibuprofen increased the probability of cough resolution (hazard ratio 1.03, 95% confidence interval 0.78 to 1.35 and 1.23, 0.93 to 1.61, respectively) compared with placebo. Adverse events were observed in 27 patients, and were more common in the antibiotic arm (12%) than ibuprofen or placebo arms (5% and 3%, respectively; P<0.01).
Conclusion: No significant differences were observed in the number of days with cough between patients with uncomplicated acute bronchitis and discoloured sputum treated with ibuprofen, amoxicillin-clavulanic acid, or placebo.
Trial registration: Current Controlled Trials ISRCTN07852892.
Conflict of interest statement
Competing interests: CL receives research grants from the European Commission (Sixth and Seventh Programme Frameworks), Catalan Society of Family Medicine, and Instituto de Salud Carlos III (Spanish Ministry of Health). Dr. Moragas reports receiving research grants from the Spanish Society of Family Medicine, Fundació Jordi Gol i Gurina, and Instituto de Salud Carlos III (Spanish Ministry of Health). Dr. Miravitlles reports receiving: honoraria for lectures from Bayer-Schering, Boehringer Ingelheim, Pfizer, Nycomed, AstraZeneca, and Novartis, payment for the development of educational presentations from Bayer-Schering, serving on the advisory boards of Bayer-Schering, Boehringer Ingelheim, Pfizer, Nycomed, GlaxoSmithKline, Almirall, AstraZeneca, and Novartis; and receiving consulting fees from Bayer-Schering, Boehringer-Ingelheim, Pfizer, Nycomed, GlaxoSmithKline, Almirall, AstraZeneca, and Novartis. The other authors do not report disclosures.
Figures
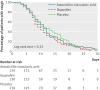

Comment in
-
Neither anti-inflammatory nor antibiotic treatment significantly shortens duration of cough in acute bronchitis compared with placebo.Evid Based Med. 2014 Jun;19(3):98. doi: 10.1136/eb-2013-101643. Epub 2014 Jan 22. Evid Based Med. 2014. PMID: 24453088 No abstract available.
-
Symptomverlauf bei Patienten mit akuter Bronchitis: Ibuprofen vs. Amoxicillin/Clavulansäure.Praxis (Bern 1994). 2014 Feb 12;103(4):233-4. doi: 10.1024/1661-8157/a001566. Praxis (Bern 1994). 2014. PMID: 24518242 German. No abstract available.
References
-
- Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al; Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect 2011;17(Suppl 6):E1-59. - PMC - PubMed
-
- Chalmers JD, Hill AT. Investigation of “non-responding” presumed lower respiratory tract infection in primary care. BMJ 2011;343:d5840. - PubMed
-
- Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Côté S, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis 2002;186:1330-4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
