Structure and function of voltage-gated sodium channels at atomic resolution
- PMID: 24097157
- PMCID: PMC3885250
- DOI: 10.1113/expphysiol.2013.071969
Structure and function of voltage-gated sodium channels at atomic resolution
Abstract
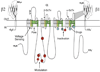
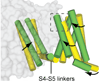
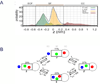
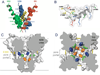
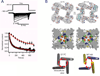
Voltage-gated sodium channels initiate action potentials in nerve, muscle and other excitable cells. Early physiological studies described sodium selectivity, voltage-dependent activation and fast inactivation, and developed conceptual models for sodium channel function. This review article follows the topics of my 2013 Sharpey-Schafer Prize Lecture and gives an overview of research using a combination of biochemical, molecular biological, physiological and structural biological approaches that have elucidated the structure and function of sodium channels at the atomic level. Structural models for voltage-dependent activation, sodium selectivity and conductance, drug block and both fast and slow inactivation are discussed. A perspective for the future envisions new advances in understanding the structural basis for sodium channel function and the opportunity for structure-based discovery of novel therapeutics.
Figures

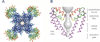
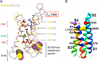


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

