Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere
- PMID: 24097350
- PMCID: PMC7449521
- DOI: 10.1038/nature12663
Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere
Abstract
Nucleation of aerosol particles from trace atmospheric vapours is thought to provide up to half of global cloud condensation nuclei. Aerosols can cause a net cooling of climate by scattering sunlight and by leading to smaller but more numerous cloud droplets, which makes clouds brighter and extends their lifetimes. Atmospheric aerosols derived from human activities are thought to have compensated for a large fraction of the warming caused by greenhouse gases. However, despite its importance for climate, atmospheric nucleation is poorly understood. Recently, it has been shown that sulphuric acid and ammonia cannot explain particle formation rates observed in the lower atmosphere. It is thought that amines may enhance nucleation, but until now there has been no direct evidence for amine ternary nucleation under atmospheric conditions. Here we use the CLOUD (Cosmics Leaving OUtdoor Droplets) chamber at CERN and find that dimethylamine above three parts per trillion by volume can enhance particle formation rates more than 1,000-fold compared with ammonia, sufficient to account for the particle formation rates observed in the atmosphere. Molecular analysis of the clusters reveals that the faster nucleation is explained by a base-stabilization mechanism involving acid-amine pairs, which strongly decrease evaporation. The ion-induced contribution is generally small, reflecting the high stability of sulphuric acid-dimethylamine clusters and indicating that galactic cosmic rays exert only a small influence on their formation, except at low overall formation rates. Our experimental measurements are well reproduced by a dynamical model based on quantum chemical calculations of binding energies of molecular clusters, without any fitted parameters. These results show that, in regions of the atmosphere near amine sources, both amines and sulphur dioxide should be considered when assessing the impact of anthropogenic activities on particle formation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




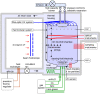



References
-
- Merikanto J, Spracklen DV, Mann GW, Pickering SJ, Carslaw KS. Impact of nucleation on global CCN. Atmos. Chem. Phys. 2009;9:8601–8616.
-
- IPCC. Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, 2007)
-
- Kirkby J, et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature. 2011;476:429–433. - PubMed
-
- Kurtén T, Loukonen V, Vehkamäki H, Kulmala M. Amines are likely to enhance neutral and ion-induced sulphuric acid-water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 2008;8:4095–4103.
-
- Loukonen V, et al. Enhancing effect of dimethylamine in sulphuric acid nucleation in the presence of water—a computational study. Atmos. Chem. Phys. 2010;10:4961–4974.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

