Alkyl hydroperoxide reductase repair by Helicobacter pylori methionine sulfoxide reductase
- PMID: 24097943
- PMCID: PMC3837964
- DOI: 10.1128/JB.01001-13
Alkyl hydroperoxide reductase repair by Helicobacter pylori methionine sulfoxide reductase
Abstract
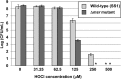
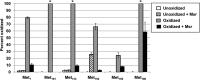
Protein exposure to oxidants such as HOCl leads to formation of methionine sulfoxide (MetSO) residues, which can be repaired by methionine sulfoxide reductase (Msr). A Helicobacter pylori msr strain was more sensitive to HOCl-mediated killing than the parent. Because of its abundance in H. pylori and its high methionine content, alkyl hydroperoxide reductase C (AhpC) was hypothesized to be prone to methionine oxidation. AhpC was expressed as a recombinant protein in Escherichia coli. AhpC activity was abolished by HOCl, while all six methionine residues of the enzyme were fully to partially oxidized. Upon incubation with a Msr repair mixture, AhpC activity was restored to nonoxidized levels and the MetSO residues were repaired to methionine, albeit to different degrees. The two most highly oxidized and then Msr-repaired methionine residues in AhpC, Met101 and Met133, were replaced with isoleucine residues by site-directed mutagenesis, either individually or together. E. coli cells expressing variant versions were more sensitive to t-butyl hydroperoxide than cells expressing native protein, and purified AhpC variant proteins had 5% to 39% of the native enzyme activity. Variant proteins were still able to oligomerize like the native version, and circular dichroism (CD) spectra of variant proteins revealed no significant change in AhpC conformation, indicating that the loss of activity in these variants was not related to major structural alterations. Our results suggest that both Met101 and Met133 residues are important for AhpC catalytic activity and that their integrity relies on the presence of a functional Msr.
Figures


References
-
- Marshall BJ, Warren JR. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311–1315 - PubMed
-
- Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328–1333 - PubMed
-
- Blaser MJ. 1995. The role of Helicobacter pylori in gastritis and its progression to peptic ulcer disease. Aliment. Pharmacol. Ther. 9(Suppl 1):27–30 - PubMed
-
- Sipponen P, Hyvarinen H, Seppala K, Blaser MJ. 1998. Review article: pathogenesis of the transformation from gastritis to malignancy. Aliment. Pharmacol. Ther. 12(Suppl 1):61–71 - PubMed
-
- Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, Park CK, Kasai H, Rhee KH. 1996. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 56:1279–1282 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

