Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility
- PMID: 24097979
- PMCID: PMC3829145
- DOI: 10.1074/jbc.M113.496802
Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility
Abstract
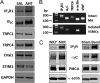
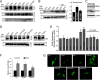
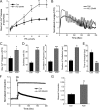
Resistance arteries show accentuated responsiveness to vasoconstrictor agonists in hypertension, and this abnormality relies partly on enhanced Ca(2+) signaling in vascular smooth muscle (VSM). Although inositol 1,4,5-triphosphate receptors (IP3Rs) are abundant in VSM, their role in the molecular remodeling of the Ca(2+) signaling machinery during hypertension has not been addressed. Therefore, we compared IP3R expression and function between mesenteric arteries of normotensive and hypertensive animals. Levels of IP3R transcript and protein were significantly increased in mesenteric arteries of hypertensive animals, and pharmacological inhibition of the IP3R revealed a higher contribution of IP3-dependent Ca(2+) release to vascular contraction in these arteries. Subsequently, we established cultured aortic VSM A7r5 cells as a cellular model that replicates IP3R up-regulation during hypertension by depolarizing the VSM cell membrane. IP3R up-regulation requires Ca(2+) influx through L-type Ca(2+) channels, followed by activation of the calcineurin-NFAT axis, resulting in IP3R transcription. Functionally, IP3R up-regulation in VSM is associated with enhancement and sensitization of IP3-dependent Ca(2+) release, resulting in increased VSM contraction in response to agonist stimulation.
Keywords: A7r5; Calcineurin; Calcium Signaling; Hypertension; IP3 Receptor; NFAT Transcription Factor; Vascular Smooth Muscle Cells.
Figures

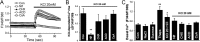
References
-
- Oparil S., Zaman M. A., Calhoun D. A. (2003) Pathogenesis of hypertension. Ann. Intern. Med. 139, 761–776 - PubMed
-
- Rizzoni D., Agabiti-Rosei E. (2012) Structural abnormalities of small resistance arteries in essential hypertension. Intern. Emerg. Med. 7, 205–212 - PubMed
-
- Lemarié C. A., Tharaux P. L., Lehoux S. (2010) Extracellular matrix alterations in hypertensive vascular remodeling. J. Mol. Cell Cardiol. 48, 433–439 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

