Structural insights into functional overlapping and differentiation among myosin V motors
- PMID: 24097982
- PMCID: PMC3837155
- DOI: 10.1074/jbc.M113.507202
Structural insights into functional overlapping and differentiation among myosin V motors
Abstract
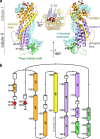
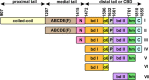
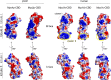
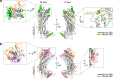
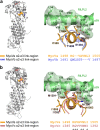
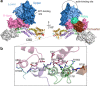
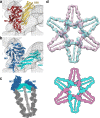
Myosin V (MyoV) motors have been implicated in the intracellular transport of diverse cargoes including vesicles, organelles, RNA-protein complexes, and regulatory proteins. Here, we have solved the cargo-binding domain (CBD) structures of the three human MyoV paralogs (Va, Vb, and Vc), revealing subtle structural changes that drive functional differentiation and a novel redox mechanism controlling the CBD dimerization process, which is unique for the MyoVc subclass. Moreover, the cargo- and motor-binding sites were structurally assigned, indicating the conservation of residues involved in the recognition of adaptors for peroxisome transport and providing high resolution insights into motor domain inhibition by CBD. These results contribute to understanding the structural requirements for cargo transport, autoinhibition, and regulatory mechanisms in myosin V motors.
Keywords: Cargo-binding Domain; Cell Signaling; Crystal Structure; Intracellular Trafficking; Molecular Motors; Molecular Plasticity; Myosin; Myosin V; Redox Dimerization.
Figures





References
-
- Desnos C., Huet S., Darchen F. (2007) “Should I stay or should I go?” Myosin V function in organelle trafficking. Biol. Cell 99, 411–423 - PubMed
-
- Hammer J. A., 3rd, Sellers J. R. (2012) Walking to work. Roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 13, 13–26 - PubMed
-
- Sellers J. R., Veigel C. (2006) Walking with myosin V. Curr. Opin. Cell Biol. 18, 68–73 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

