A conserved protein motif is required for full modulatory activity of negative elongation factor subunits NELF-A and NELF-B in modifying glucocorticoid receptor-regulated gene induction properties
- PMID: 24097989
- PMCID: PMC3837147
- DOI: 10.1074/jbc.M113.512426
A conserved protein motif is required for full modulatory activity of negative elongation factor subunits NELF-A and NELF-B in modifying glucocorticoid receptor-regulated gene induction properties
Abstract
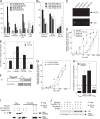
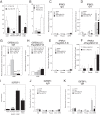
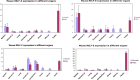
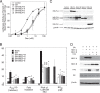
NELF-B is a BRCA1-interacting protein and subunit (with NELF-A, -C/D, and -E) of the human negative elongation factor (NELF) complex, which participates in RNA polymerase II pausing shortly after transcription initiation, especially for synchronized gene expression. We now report new activities of NELF-B and other NELF complex subunits, which are to attenuate glucocorticoid receptor (GR)-mediated gene induction, reduce the partial agonist activity of an antagonist, and increase the EC50 of an agonist during nonsynchronized expression of exogenous and endogenous reporters. Stable knockdown of endogenous NELF-B has the opposite effects on an exogenous gene. The GR ligand-binding domain suffices for these biological responses. ChIP assays reveal that NELF-B diminishes GR recruitment to promoter regions of two endogenous genes. Using a new competition assay, NELF-A and NELF-B are each shown to act independently as competitive decelerators at two steps after the site of GR action and before or at the site of reporter gene activity. A common motif in each NELF was identified that is required for full activity of both NELF-A and NELF-B. These studies allow us to position the actions of two new modulators of GR-regulated transactivation, NELF-A and NELF-B, relative to other factors in the overall gene induction sequence.
Keywords: Cofactor Action; Competitive Decelerator; EC50; Gene Induction; Gene Transcription; Glucocorticoid Receptor; Mathematical Modeling; NELF-A and NELF-B; Steroid Hormone; Transcription Factors.
Figures



References
-
- Simons S. S., Jr. (2003) The importance of being varied in steroid receptor transactivation. Trends Pharmacol. Sci. 24, 253–259 - PubMed
-
- Kunz S., Sandoval R., Carlsson P., Carlstedt-Duke J., Bloom J. W., Miesfeld R. L. (2003) Identification of a novel glucocorticoid receptor mutation in budesonide-resistant human bronchial epithelial cells. Mol. Endocrinol. 17, 2566–2582 - PubMed
-
- Zhao Q., Pang J., Favata M. F., Trzaskos J. M. (2003) Receptor density dictates the behavior of a subset of steroid ligands in glucocorticoid receptor-mediated transrepression. Int. Immunopharmacol. 3, 1803–1817 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

