NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality
- PMID: 24098114
- PMCID: PMC3789746
- DOI: 10.1371/journal.ppat.1003634
NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality
Abstract
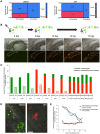
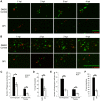
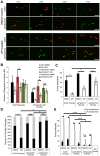
Candida albicans is a human commensal and clinically important fungal pathogen that grows as both yeast and hyphal forms during human, mouse and zebrafish infection. Reactive oxygen species (ROS) produced by NADPH oxidases play diverse roles in immunity, including their long-appreciated function as microbicidal oxidants. Here we demonstrate a non-traditional mechanistic role of NADPH oxidase in promoting phagocyte chemotaxis and intracellular containment of fungi to limit filamentous growth. We exploit the transparent zebrafish model to show that failed NADPH oxidase-dependent phagocyte recruitment to C. albicans in the first four hours post-infection permits fungi to germinate extracellularly and kill the host. We combine chemical and genetic tools with high-resolution time-lapse microscopy to implicate both phagocyte oxidase and dual-specific oxidase in recruitment, suggesting that both myeloid and non-myeloid cells promote chemotaxis. We show that early non-invasive imaging provides a robust tool for prognosis, strongly connecting effective early immune response with survival. Finally, we demonstrate a new role of a key regulator of the yeast-to-hyphal switching program in phagocyte-mediated containment, suggesting that there are species-specific methods for modulation of NADPH oxidase-independent immune responses. These novel links between ROS-driven chemotaxis and fungal dimorphism expand our view of a key host defense mechanism and have important implications for pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

