APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host
- PMID: 24098115
- PMCID: PMC3789815
- DOI: 10.1371/journal.ppat.1003641
APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host
Abstract
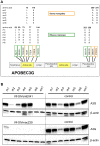
Cellular restriction factors, which render cells intrinsically resistant to viruses, potentially impose genetic barriers to cross-species transmission and emergence of viral pathogens in nature. One such factor is APOBEC3G. To overcome APOBEC3G-mediated restriction, many lentiviruses encode Vif, a protein that targets APOBEC3G for degradation. As with many restriction factor genes, primate APOBEC3G displays strong signatures of positive selection. This is interpreted as evidence that the primate APOBEC3G locus reflects a long-term evolutionary "arms-race" between retroviruses and their primate hosts. Here, we provide direct evidence that APOBEC3G has functioned as a barrier to cross-species transmission, selecting for viral resistance during emergence of the AIDS-causing pathogen SIVmac in captive colonies of Asian macaques in the 1970s. Specifically, we found that rhesus macaques have multiple, functionally distinct APOBEC3G alleles, and that emergence of SIVmac and simian AIDS required adaptation of the virus to evade APOBEC3G-mediated restriction. Our evidence includes the first comparative analysis of APOBEC3G polymorphism and function in both a reservoir and recipient host species (sooty mangabeys and rhesus macaques, respectively), and identification of adaptations unique to Vif proteins of the SIVmac lineage that specifically antagonize rhesus APOBEC3G alleles. By demonstrating that interspecies variation in a known restriction factor selected for viral counter-adaptations in the context of a documented case of cross-species transmission, our results lend strong support to the evolutionary "arms-race" hypothesis. Importantly, our study confirms that APOBEC3G divergence can be a critical determinant of interspecies transmission and emergence of primate lentiviruses, including viruses with the potential to infect and spread in human populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Charleston MA, Robertson DL (2002) Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Systematic biology 51: 528–535. - PubMed
-
- Hahn BH, Shaw GM, De Cock KM, Sharp PM (2000) AIDS as a zoonosis: scientific and public health implications. Science 287: 607–614. - PubMed
-
- Apetrei C, Robertson DL, Marx PA (2004) The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Frontiers in bioscience: a journal and virtual library 9: 225–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

