Contribution of host intracellular transport machineries to intercellular movement of turnip mosaic virus
- PMID: 24098128
- PMCID: PMC3789768
- DOI: 10.1371/journal.ppat.1003683
Contribution of host intracellular transport machineries to intercellular movement of turnip mosaic virus
Abstract
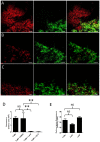
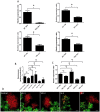
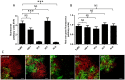
The contribution of different host cell transport systems in the intercellular movement of turnip mosaic virus (TuMV) was investigated. To discriminate between primary infections and secondary infections associated with the virus intercellular movement, a gene cassette expressing GFP-HDEL was inserted adjacent to a TuMV infectious cassette expressing 6K₂:mCherry, both within the T-DNA borders of the binary vector pCambia. In this system, both gene cassettes were delivered to the same cell by a single binary vector and primary infection foci emitted green and red fluorescence while secondarily infected cells emitted only red fluorescence. Intercellular movement was measured at 72 hours post infiltration and was estimated to proceed at an average rate of one cell being infected every three hours over an observation period of 17 hours. To determine if the secretory pathway were important for TuMV intercellular movement, chemical and protein inhibitors that blocked both early and late secretory pathways were used. Treatment with Brefeldin A or Concanamycin A or expression of ARF1 or RAB-E1d dominant negative mutants, all of which inhibit pre- or post-Golgi transport, reduced intercellular movement by the virus. These treatments, however, did not inhibit virus replication in primary infected cells. Pharmacological interference assays using Tyrphostin A23 or Wortmannin showed that endocytosis was not important for TuMV intercellular movement. Lack of co-localization by endocytosed FM4-64 and Ara7 (AtRabF2b) with TuMV-induced 6K₂-tagged vesicles further supported this conclusion. Microfilament depolymerizing drugs and silencing expression of myosin XI-2 gene, but not myosin VIII genes, also inhibited TuMV intercellular movement. Expression of dominant negative myosin mutants confirmed the role played by myosin XI-2 as well as by myosin XI-K in TuMV intercellular movement. Using this dual gene cassette expression system and transport inhibitors, components of the secretory and actomyosin machinery were shown to be important for TuMV intercellular spread.
Conflict of interest statement
The Samuel Roberts Noble Foundation is an independent, nonprofit institute headquartered in Ardmore, Oklahoma. Founded in 1945, the Noble Foundation conducts direct operations, including assisting farmers and ranchers, and conducting plant science research and agricultural programs, to enhance agricultural productivity regionally, nationally and internationally. The Foundation has no competing interests influencing the other authors (i.e. they support only work of RSN as an employee). The funder, Noble Foundation, did not influence the study design, collection, analysis and interpretation of data, writing of the paper or the decision to submit the manuscript for publication. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures





References
-
- Maule AJ, Benitez-Alfonso Y, Faulkner C (2011) Plasmodesmata - membrane tunnels with attitude. Curr Opin Plant Biol 14: 683–690. - PubMed
-
- Niehl A, Heinlein M (2011) Cellular pathways for viral transport through plasmodesmata. Protoplasma 248: 75–99. - PubMed
-
- Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ (2010) Plasmodesmata: gateways to local and systemic virus infection. Mol Plant Microbe Interact 23: 1403–1412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

