Identification of 526 conserved metazoan genetic innovations exposes a new role for cofactor E-like in neuronal microtubule homeostasis
- PMID: 24098140
- PMCID: PMC3789837
- DOI: 10.1371/journal.pgen.1003804
Identification of 526 conserved metazoan genetic innovations exposes a new role for cofactor E-like in neuronal microtubule homeostasis
Abstract
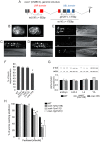
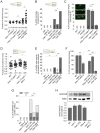
The evolution of metazoans from their choanoflagellate-like unicellular ancestor coincided with the acquisition of novel biological functions to support a multicellular lifestyle, and eventually, the unique cellular and physiological demands of differentiated cell types such as those forming the nervous, muscle and immune systems. In an effort to understand the molecular underpinnings of such metazoan innovations, we carried out a comparative genomics analysis for genes found exclusively in, and widely conserved across, metazoans. Using this approach, we identified a set of 526 core metazoan-specific genes (the 'metazoanome'), approximately 10% of which are largely uncharacterized, 16% of which are associated with known human disease, and 66% of which are conserved in Trichoplax adhaerens, a basal metazoan lacking neurons and other specialized cell types. Global analyses of previously-characterized core metazoan genes suggest a prevalent property, namely that they act as partially redundant modifiers of ancient eukaryotic pathways. Our data also highlights the importance of exaptation of pre-existing genetic tools during metazoan evolution. Expression studies in C. elegans revealed that many metazoan-specific genes, including tubulin folding cofactor E-like (TBCEL/coel-1), are expressed in neurons. We used C. elegans COEL-1 as a representative to experimentally validate the metazoan-specific character of our dataset. We show that coel-1 disruption results in developmental hypersensitivity to the microtubule drug paclitaxel/taxol, and that overexpression of coel-1 has broad effects during embryonic development and perturbs specialized microtubules in the touch receptor neurons (TRNs). In addition, coel-1 influences the migration, neurite outgrowth and mechanosensory function of the TRNs, and functionally interacts with components of the tubulin acetylation/deacetylation pathway. Together, our findings unveil a conserved molecular toolbox fundamental to metazoan biology that contains a number of neuronally expressed and disease-related genes, and reveal a key role for TBCEL/coel-1 in regulating microtubule function during metazoan development and neuronal differentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Michod RE, Viossat Y, Solari CA, Hurand M, Nedelcu AM (2006) Life-history evolution and the origin of multicellularity. J Theor Biol 239: 257–272. - PubMed
-
- Rokas A (2008) The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu Rev Genet 42: 235–251. - PubMed
-
- Ruiz-Trillo I, Burger G, Holland PW, King N, Lang BF, et al. (2007) The origins of multicellularity: a multi-taxon genome initiative. Trends Genet 23: 113–118. - PubMed
-
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, et al. (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317: 86–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

