rac-Methyl (3aR*,4S*,5R*,7aR*)-5,7a-bis-(acet-yloxy)-3-oxo-2-phenyl-octa-hydro-1H-iso-indole-4-carboxyl-ate
- PMID: 24098237
- PMCID: PMC3790418
- DOI: 10.1107/S1600536813025129
rac-Methyl (3aR*,4S*,5R*,7aR*)-5,7a-bis-(acet-yloxy)-3-oxo-2-phenyl-octa-hydro-1H-iso-indole-4-carboxyl-ate
Abstract
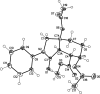
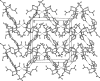
The title molecule, C20H23NO7, the product of nucleophilic cleavage of the 3a,6-ep-oxy bridge in 1-oxo-2-phenyl-octa-hydro-3a,6-ep-oxy-iso-indole-7-carboxyl-ate, comprises a cis-fused bicyclic system containing a 2-pyrrolidinone ring in an envelope conformation (with the C atom bearing the carboxyl-ate substituent as the flap) and a cyclo-hexane ring in a chair conformation. The carboxyl-ate substituent occupies the equatorial position, whereas the two acet-yloxy substituents are in axial positions. The N atom has a trigonal-planar geometry, the sum of the bond angles being 359.3 (3)°. The dihedral angle between the mean plane of the four planar atoms of the pyrrolidinone ring and the phenyl ring is 25.98 (6)°. In the crystal, mol-ecules are linked into zigzag chains along the c-axis direction by C-H⋯O hydrogen bonds.
Figures
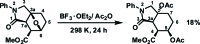
References
-
- Bruker (2001). SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2003). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Claeys, D. D., Stevens, C. V., Roman, B. I., Caveye, P. van D., Waroquier, M. & Speybroeck, V. V. (2010). Org. Biomol. Chem. 8, 3644–3654. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
