Di-hydro-cyclam dimaleate [H2(cyclam)(maleate)2]
- PMID: 24098252
- PMCID: PMC3790433
- DOI: 10.1107/S1600536813025580
Di-hydro-cyclam dimaleate [H2(cyclam)(maleate)2]
Abstract
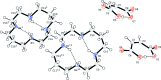
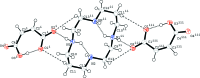
The asymmetric unit of the title mol-ecular salt [systematic name: 1,4,8,11-tetraazacyclotetradecane-1,8-diium bis(3-carboxy-prop-2-enoate)], C10H26N4 (2+)·2C4H3O4 (-), contains two half-cations (both completed by crystallographic inversion symmetry) and two maleate anions. The cyclam macrocycles adopt trans-III conformations, supported by two intra-molecular N-H⋯O hydrogen bonds. The O-bonded H atom of each maleate ion is disordered over two positions with an occupancy ratio of 0.61 (5):0.39 (5): each one generates an intra-molecular O-H⋯O hydrogen bond. In the crystal, the cations are linked to the anions by N-H⋯O hydrogen bonds, generating [001] chains.
Figures

References
-
- Bandoli, G., Dolmella, A. & Gatto, S. (1993). J. Crystallogr. Spectrosc. Res. 23, 755–758.
-
- Bosnich, B., Poon, C. K. & Tobe, M. L. (1965). Inorg. Chem. 4, 1102–1108.
-
- Bruker (2005). SADABS, SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Dale, J. (1973). Acta Chem. Scand. 27, 1115–1129.
-
- Dale, J. (1976). Top. Stereochem. 9, 199–270.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
